Cortical flow and the establishment of axial polarization in the C. elegans embryo is generated by the contraction of the actomyosin meshwork at the cortex. Evidence for the co-localization of actin and myosin at the foci of this cortical meshwork is shown below in an embryo co-stained for filamentous actin and myosin. Areas of co-localization appear as bright white spots and are seen only at foci. This data is consistent with observations reported by Ed Munro in his unpublished data. The hypothesis that actin and myosin interact in some way to generate cortical flow is supported by their co-localization at the foci. The implications of this is that in order to understand how cortical flow is regulated during the establishment of asymmetry in the early embryo we need to look at the distribution of both actin and myosin.
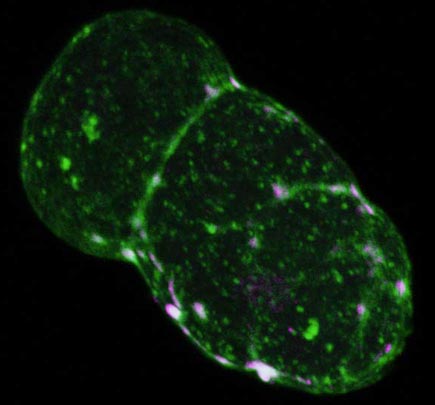
Wild type C. elegans embryo at pseudocleavage. The actomyosin cap is contracting towards the anterior (lower right). Embryo is stained for F-actin (green) and myosin (magenta). Points of co-localization appear white and are located at the foci of the cortical meshwork. This image has an associated movie available in two sizes. The movies will open in a new window sized as indicated. View the smaller movie [new window, 540x620 pixels, 2.8 MB], or larger image [new window, 660x780 pixels, 5.4 MB].
In the previous section on myosin dynamics we showed evidence that PAR-3 and MEX-5/6 modulate the cortical flow of myosin. However, in order to fully understand the nature of the feedback loop between PAR-3, MEX-5/6, and the cortex we need to also look specifically at the distribution of actin in wild type and PAR-3 and MEX-5/6 mutants during cortical contraction and cell polarization. To do this we fixed embryos on slides and stained them with phalloidin, which binds specifically to filamentous actin. We fixed and stained three groups of embryos: wild-type, embryos lacking MEX-5/6, and embryos lacking PAR-3. All embryos were fixed and scanned with the Radiance 2000 confocal microscope on the same day. This allowed us to make an accurate comparison of actin distribution in wild type and mutant embryos. Three specific developmental time points in prior to first cleavage were established as reference points for comparison and analysis on our images:
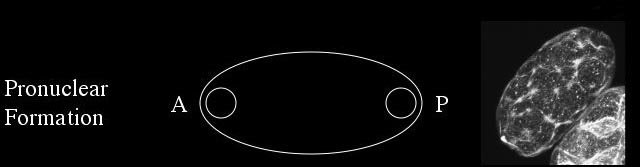
During pronuclear formation the male and female pronuclei are visible at opposite poles of the embryo and a cortical meshwork of actin forms at the cortex.
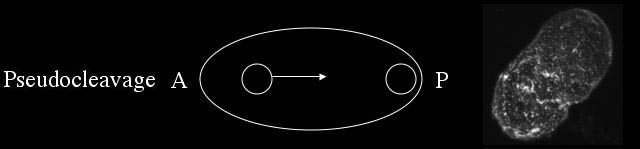
Pseudocleavage is characterized by the migration of the female pronucleus to the posterior end of the cell, the formation of a pseaudocleavage furrow, and the local weakening and contraction of the cortical meshwork to the anterior.
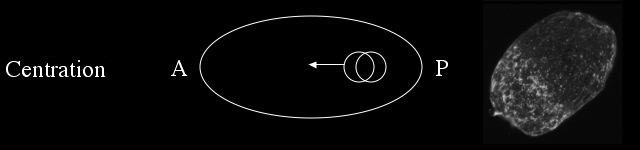
During centration the male and female pronuclei migrate to the center of the embryo in preparation for cleavage and the cortical meshwork forms a cap at the anterior.
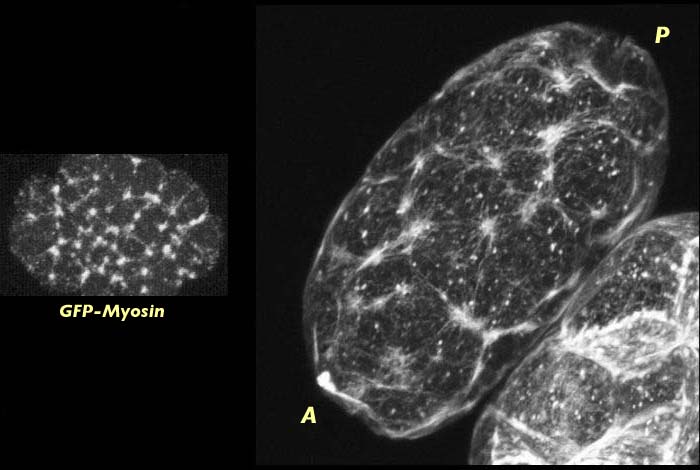
Wild type C. elegans embryo at pronuclear formation stained for actin (right). Meshwork of actin foci cover entire embryo. Distribution of actin in fixed embryos is the same as for GFP-myosin visualized in live embryos, as shown on the right. The actin stained image has an associated 3-D reconstruction movie available in two sizes. The movies will open in a new window sized as indicated. View the smaller movie [new window, 400x540 pixels, 840 KB], or larger image [new window, 600x760 pixels, 2.8 MB].
The organization and distribution of the actin meshwork during pronuclear formation is very similar to that of the myosin meshwork at this same stage in development. This result is not surprising given that actin and myosin have been shown to co-localize at the foci of the cortical meshwork. Since myosin exerts force on filamentous actin we can hypothesize that myosin may be responsible for organizing actin into this interconnected meshwork of foci. Regardless of the mechanism, however, it appears from our data on actin distribution in mutant embryos that some organization of actin into a network of foci is required for cortical contraction and flow to occur.
At pseudocleavage actin foci cap to the anterior end of the embryo:
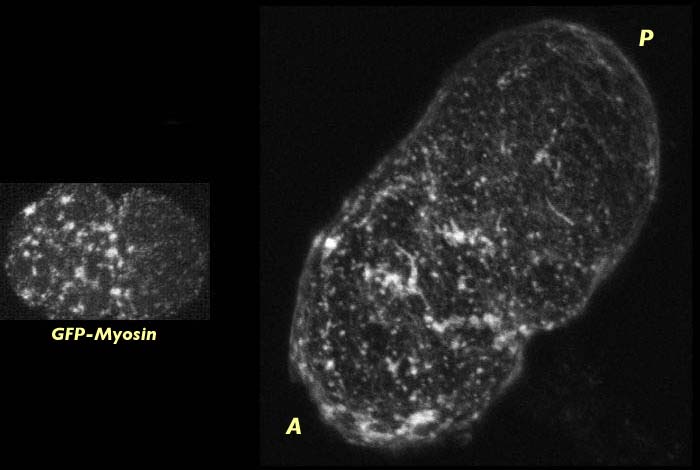
Wild type C. elegans embryo at late pseudocleavage stained for actin (right). Actin is capping to the anterior end of the embryo. Myosin distribution at this stage can be seen on the left. The actin stained image has an associated 3-D reconstruction movie available in two sizes. The movies will open in a new window sized as indicated. View the smaller movie [new window, 400x540 pixels, 670 KB], or larger image [new window, 600x760 pixels, 2.8 MB].
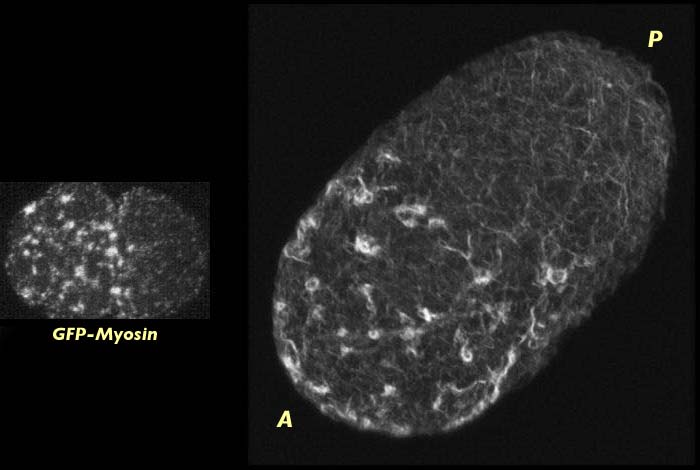
Wild type C. elegans embryo at the end of pseudocleavage stained for actin (right). Actin has capped to the anterior end of the embryo. The progression of action to the anterior can be see by comparing this image to the one above which is a slightly earlier stage. Myosin distribution at this the end of pseudocleavage can be seen on the left. The actin stained image has an associated 3-D reconstruction movie available in two sizes. View the smaller movie [new window, 400x600 pixels, 670 KB], or larger image [new window, 640x820 pixels, 2.8 MB].
This global movement of actin occurs at the same time that the myosin meshwork is contracting towards the anterior which suggests that the co-localization of actin and myosin persists through pseudocleavage as the cortical meshwork contracts. We cannot determine whether actin is being pulled towards the anterior by the contraction and flow of the myosin meshwork or whether the actin is actively participating in cortical contraction, but it is presumably the latter.
By centration actin is localized at the anterior end of the embryo and where it forms a dense actin cap:
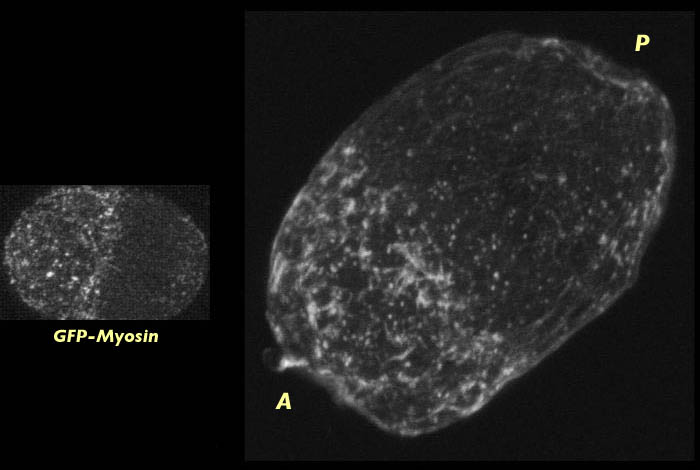
Wild-type C. elegans embryo at centration stained for actin (right). The fine meshwork of myosin which forms ater pseudocleavage when the myosin foci disassemble can be seen on the left. The actin stained image has an associated 3-D reconstruction movie available in two sizes. View the smaller movie [new window, 400x510 pixels, 610 KB], or larger image [new window, 600x720 pixels, 2.3 MB].
Like actin, myosin forms an anterior cap at centration but the myosin foci have diassembled into a fine meshwork at this stage. Breakdown of the actin foci at centration is not apparent in our images (although they change in appearance somewhat). This suggests that actin has a slower disassembly rate than myosin and so the actin foci remain intact past the time that the myosin foci have disassembled. The density of actin at the cap at centration is likely a product of the actin foci being compressed at the anterior end of the embryo.
Embryos lacking MEX-5/6 function were obtained from worms that had been fed Mex-5/6 double-stranded RNA and were not expressing MEX-5/6 in their gonads at the time of fixation.
In embryos lacking MEX-5/6 the actin focal meshwork is not as dense as in wild-type, and unlike wild-type it does not cap to the anterior at pseudocleavage. This difference can be seen in the images below. There are a few foci towards the anterior of the MEX-5/6-deficient embryo, but they are not as dense as the foci appear in wild-type.
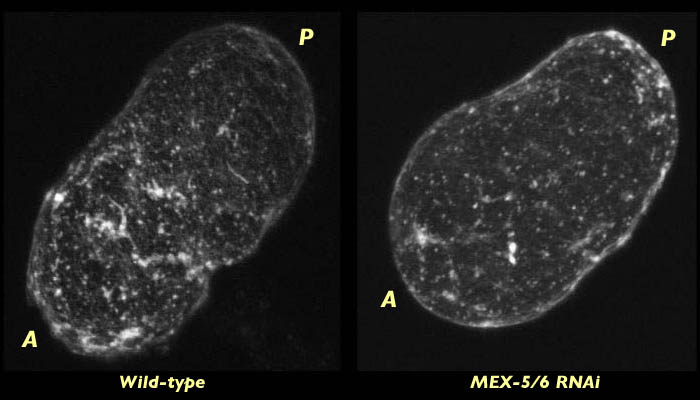
C. elegans embryo lacking MEX-5/6 fixed at pseudocleavage and stained for actin (right). Compare to wild type embryo at the same stage (left). The actin meshwork is less dense in MEX-5/6 and the capping that can be seen in wild type is not present. The MEX-5/6-deficient embryo is compared with the wild-type embryo in associated 3-D reconstruction movies available in two sizes. View the smaller movie [new window, 660x500 pixels, 1.2 MB], or the larger movie [new window, 1150x820 pixels, 4.4 MB].
By centration, however, a dense actin cap has formed at the anterior end of embryos lacking MEX-5/6. This cap does not retract as far towards the anterior as it would in wild type but is otherwise is similar to the actin cap seen wild type embryos at this stage.
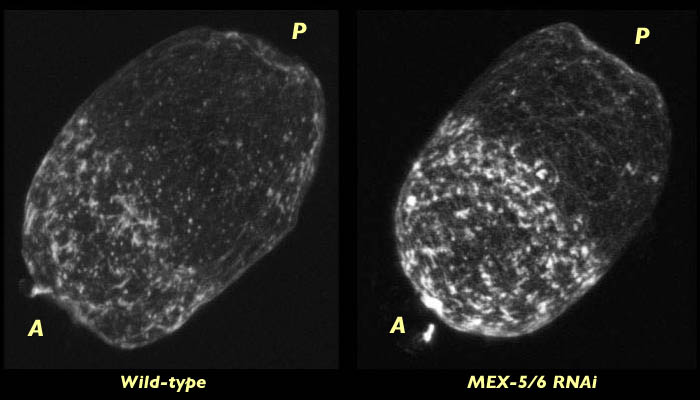
C. elegans embryo lacking MEX-5/6 fixed at centration and stained for actin (right). Compare to wild type embryo at the same stage (left). Dense actin caps have formed in both embryos at this stage. The MEX-5/6-deficient embryo is compared with the wild-type embryo in associated 3-D reconstruction movies available in two sizes. View the smaller movie [new window, 660x500 pixels, 1.2 MB], or the larger movie [new window, 1150x820 pixels, 5.1 MB].
The late contraction of the actin cap may be explained by a time-lapse movie of myosin dynamics in embryos lacking MEX-5/6. This movies show that the contraction of the cortical meshwork does not occur until after pseudocleavage when the foci have already broken down.
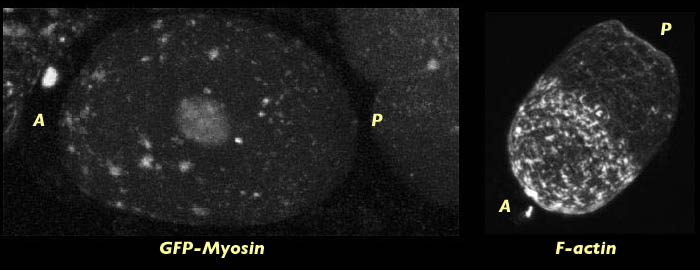
Still frame of a time lapse movie of myosin cortical contraction in a C. elegans embryo lacking MEX-5/6. Note the similar localization of the fine meshwork myosin cap that forms in the movie to the actin cap shown in the fixed actin stained embryo (right). Click the image to view a time lapse movie [new window, 600x570 pixels, 990 KB], of cortical contraction in a myosin labeled embryo.
Given that there is little cortical flow in Mex-5/6 embryos until after pseudocleavage it is not surprising that the actin does not cap at pseudocleavage. Formation of a defined actin cap at centration can be correlated to the flow of myosin to the anterior after pseaudoclevage in embryos lacking Mex-5/6.
Embryos lacking PAR-3 were obtained from a PAR-3 mutant strain of C. elegans. Fixing and staining of PAR-3 mutant embryos at nuclear formation indicated that, unlike wild-type, they do not form a contractile actin meshwork at pronuclear formation.
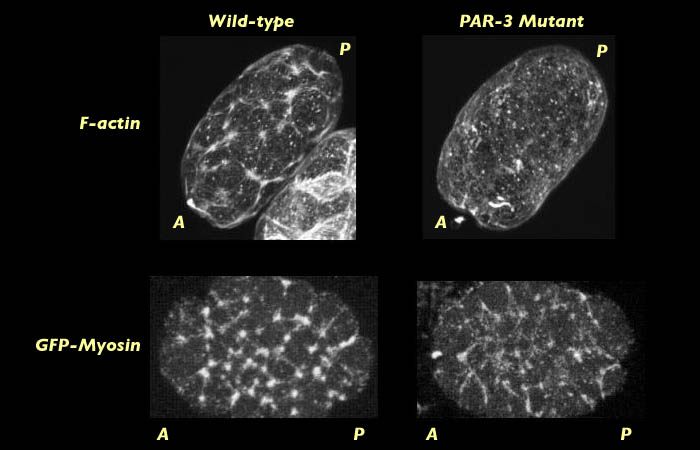
Comparison of actin and myosin distribution on wild type and PAR-3 mutant C. elegans at pronuclear formation. 3-D reconstruction movies will open in new windows sized as indicated. View the smaller movie [new window, 660x570 pixels, 1.6 MB], or the larger movie [new window, 1150x800 pixels, 5.4 MB].
Actin distribution was compared to myosin distribution at pronuclear formation in PAR-3 mutants labeled for myosin. We found that myosin does form a contractile network of foci in PAR-3 mutants (See images above). This raises the question of why there is a difference in the behavior of actin and myosin at the cortex and why it is that actin fails to form a network of foci. One hypothesis in that PAR-3 may act to increase the force that myosin can exert on actin or conversely to decrease the strength by which actin resists the pulling force of myosin. Either way, we would expect to see a failure of myosin to exert enough force on the dense actin filaments to recruit them into the contractile network. This result may provide evidence for why it is that global cortical flow and the myosin capping fail to occur in live images of PAR-3 mutant embryos labeled with myosin GFP.
By centration we see that no actin cap has formed in PAR-3 mutant embryos, which is consistent with the lack of cortical contraction of the myosin meshwork observed in PAR-3 mutant embryos expressing GFP-myosin.
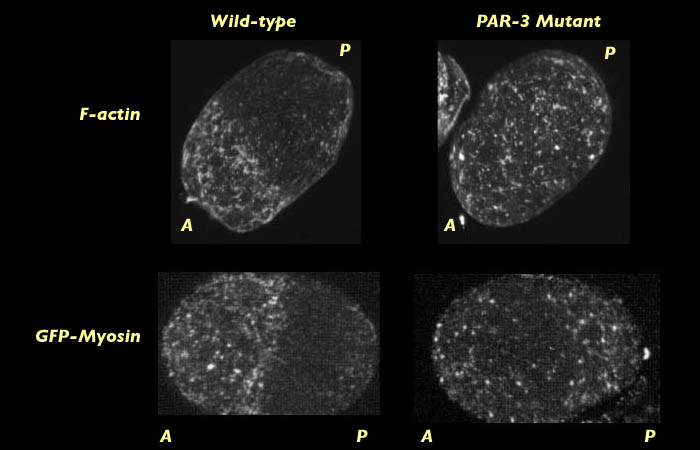
Comparison of actin and myosin distribution on wild type and PAR-3 mutant C. elegans at centration. 3-D reconstruction movies will open in new windows sized as indicated. View the smaller movie [new window, 660x570 pixels, 1.3 MB], or the larger movie [new window, 1150x800 pixels, 4.9 MB].
The result that in PAR-3 mutants myosin fails organize actin into a contractile focal meshwork supports our hypothesis that PAR-3 enhances cortical contraction. More data needs to be collected however before strong conclusions can be drawn about the specific effect of PAR-3 on the actomyosin meshwork.
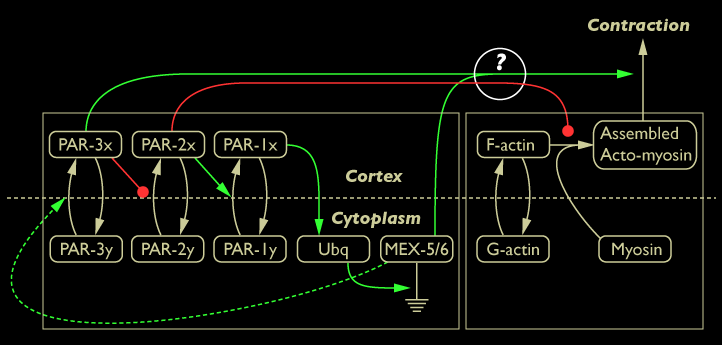
Diagram of the network model with PAR-3 labeled as enhancing cortical contraction