In order to better understand how cells move and change shape three dimensionally, we collected stacks of images of live embryos at successive time points and we made from these stacks time-lapse videos in which we chose a single focal plane from each stack, selected so we could follow a particular feature of interest. The video summarized below follows the apical surfaces of the endoderm cells. At the beginning of the video we see four endoderm cells in focus (A7.1 and A7.2 in each half of the embryo), but as these four cells invaginate more cells come into view until we can see the whole sheet of endoderm cells first flatten, then invaginate deeper into the embryo.
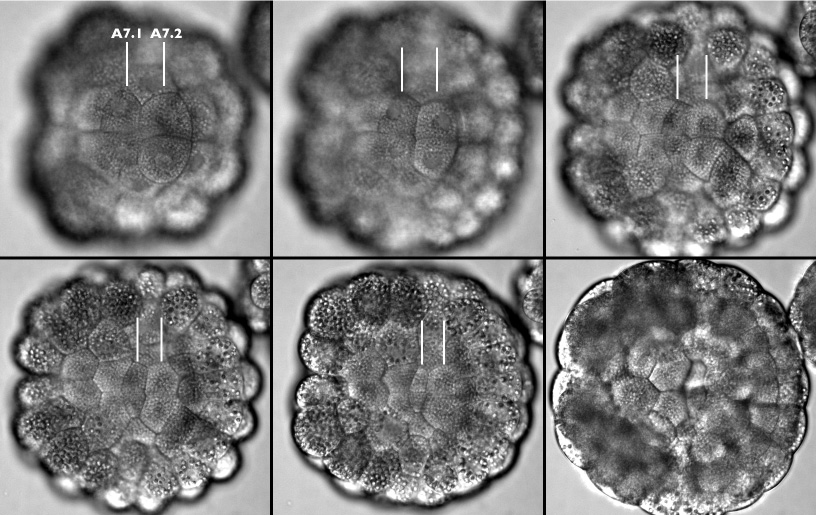
Frames from a time-lapse movie following the apices of the A7.1 and A7.2 cells through endoderm invagination (lines point out these cells on the embryo's left side). In this video we are taking a bird's-eye view onto the vegetal hemisphere, posterior is left and anterior is right. At the anterior of the vegetal hemisphere are four notochord precursor cells and four neural plate precursors (out of focus) which divide as invagination begins, and at the posterior of are several large darker cells containing many large, pigmented yolk granules, which give rise to the larval muscle. These cells are orange in a live embryo, which makes them a great aid for orienting embryos under the microscope. This movie is available in three sizes. The movies will open in a new window sized as indicated. View the small version of the movie [600x680 pixels, 1.2 MB], or a larger version [720x760 pixels, 2.2 MB], or the full-frame version [950x840 pixels, 4.5 MB].
Notice that the apical surface area of all the endoderm cells gets smaller, and that the apices of the A7.1 pair shrink the most. These two cells happen to be the ones that dive the deepest into the embryo. Also notice that the nuclei of the endoderm cells are in focus at the beginning of the video but come out of focus as these cells invaginate. As apical surfaces of endoderm get smaller, the nuclei disappear as they move deeper within each cell. Once the sheet of endoderm cells has invaginated, these cells will form a bowl that is embedded in the embryo and we can no longer focus on the apices clearly. To illustrate the change in apical surface area, we traced cell boundaries from videos like the one above:
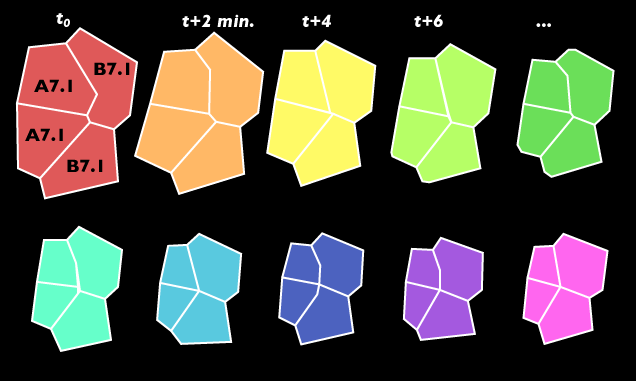
The first tracing above corresponds to the A7.1 & B7.1 cells' apical surface area shortly after invagination begins, and the last before invagination and the last tracing corresponds to the cells' apical boundary after invagination has taken place. Coloring orders timepoints along the rainbow.
We also traced cross-sectional profiles of embryos from confocal microscope images we made at different stages of invagination. We were interested to see cell deformations and the descent of the nuclei as these endoderm cells migrate down into the embryo:
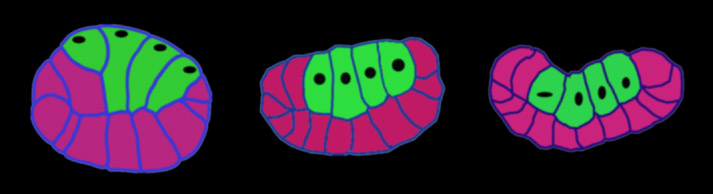
Traced cross-sections of Boltenia gastrulas, from confocal stacks stained with phalloidin to reveal cell outlines. Vegetal is up, anterior to the left, posterior to the right, in all cases. Endoderm cells are filled in green, black ovals show positions of nuclei.
The first embryo above is just beginning invagination. Notice that the nuclei of the endoderm cells are right up to the apical surface beneath the plasma membrane, and that the basal ends of the cells are much narrower than the apical ends. The middle embryo has started to invaginate; notice that the vegetal plate is much flatter than in the previous stage. As invagination begins, the nuclei start to make their descent, and another noticeable feature is that the basal surfaces are now wider than the apical surfaces of these endoderm cells. In the right most embryo, we see that once endoderm invagination is complete the apical surfaces are much narrower than the basal surfaces, and that the embryo has transformed from a ball to a bowl.
The stereo figures below are projected confocal stacks in which we manually cut away all but the ten endoderm cells in each section of the stack before projecting them:
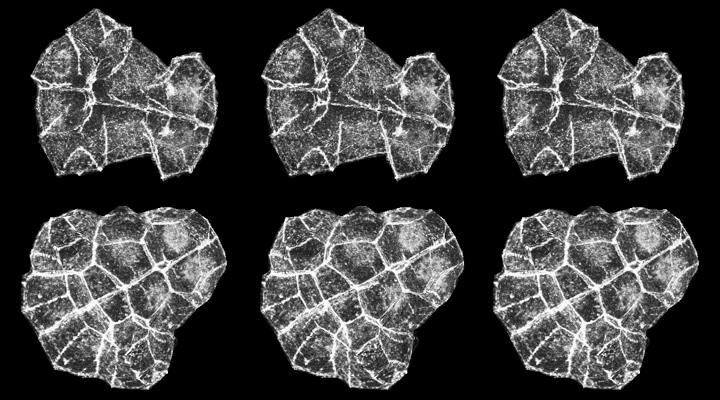
Endoderm cells before invagination (top row) and after invagination (bottom row). These figures are stereo triplets - if you don't know how to fuse stereo images, click the image to see the 3-D reconstructions as movies [new window, 950x630 pixels, 8.6 MB]. Divergent viewing: the left and middle image will fuse to yield an view in which the apical surfaces face out, whereas the middle and right image yield a veiw from the basal surface. Cross-eyed viewing yields a basal view on the left and an apical view on the right.
These 3-D reconstructions from before and after invagination emphasize the concerted change in the shapes of individual endoderm cells: the apical surface is smaller than the basal surface of endoderm cells in the top row above, but after invagination the apical surface is much smaller than the basal surface. Despite this, cells don't exchange neighbors as they invaginate, and don't even appear to shear past one another at all.
One of the principle ways in which cells generate pulling forces is by actin-based contractions. We studied the organization of actin in fixed embryos stained with phalloidin, which specifically labels filamentous actin. We expected the actin organization in cells to help us distinguish between various candidate mechanisms for invagination; for example, if purse-string contraction around the apical ends drives invagination, we would expect to see a large concentration of actin at the apical surface of these embryos, whereas if cells crawl over each other to invaginate we would expect to see actin-rich cellular extensions.
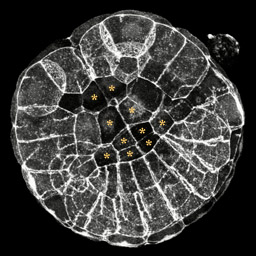
This is a projection of a 110-cell embryo in the midst of invagination. In this embryo the vegetal plate has flattened but the endoderm cells still have a ways to go. This 3-D reconstruction is available in three sizes. The movies will open in a new window sized as indicated. View the small version of the movie [500x500 pixels, 470 KB], or a larger version [600x600 pixels, 1 MB], or the full-frame version [760x760 pixels, 4.2 MB]. Posterior is to the upper left, endoderm cells are marked with asterisks.
The image above is a 3-D reconstruction of a confocal stack through a 110-cell stage embryo that has started to invaginate. It appears that there is a bright ring of actin around the apices of the endoderm, but this is not brighter then the concentration of actin around the apices of other cells or between the lateral edges of the cells. This was consistently observed in embryos fixed during and after invagination. Although this observation does not rule out the apical constriction mechanism, it does suggest that perhaps some other mechanism drives invagination.
To explore whether endoderm cells crawl upon each other or upon neighboring cells during invagination, we also looked carefully at the distribution of actin at the basal surface of the endoderm cells:
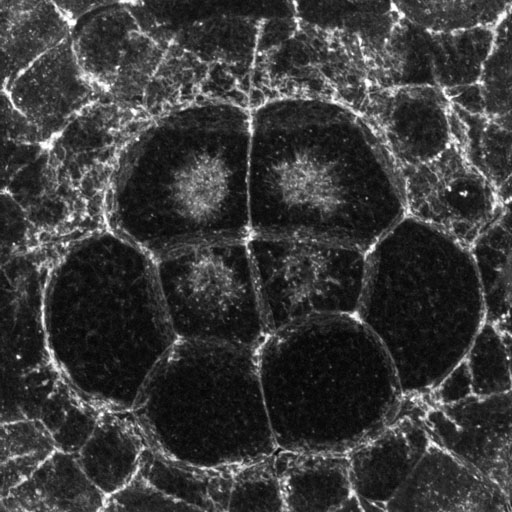
Basal region of endoderm cells in a late-invagination-stage embryo. Click the image to see a larger version [new window, 1080x800 pixels, 284 KB].
The image above is a high-magnification confocal image (projection of about a dozen serial 0.5 micron sections) showing the basal region of eight endoderm cells (the darker cells in the center). The basal-most surface is left out of this projection for clarity. There is a mesh of fine actin-rich projections that fills the spaces between the endoderm and the ectoderm, looking almost like a layer of Velcro, but it is not clear whether these actin-rich projections are part of the endoderm or the ectoderm cells or both.
To get a better idea about where the basal actin protrusions are generated, we isolated vegetal half-embryos, fixed them, and stained with phalloidin. We looked at the basal surface of the endoderm cells and did not see the Velcro-like layer of fine actin-rich protrusions. This indicates that actin-rich extensions found at the basal end of the vegetal plate either must be features of the ectoderm cells, or could be made by the endoderm cells only when in proximity to other cells. In any case, the actin distribution at the basal surface of endoderm must not be the machinery necessary for driving invagination, since isolated vegetal plates undergo invagination (see next page).
In summary, we did not find anything suggestive about actin organization either at the apical or basal surfaces of the endoderm cells. However we did notice a prominent accumulation of internal actin associated with the centrosomes:
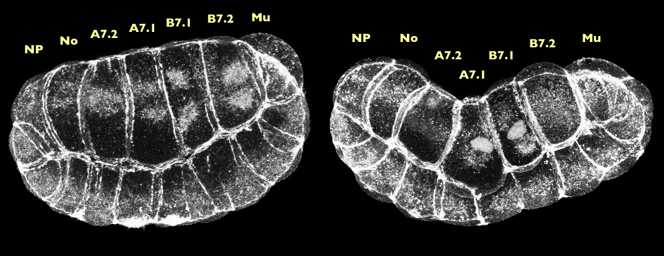
Projections of approx. 20 confocal sections, near-sagittal such that we see anterior at the right, vegetal up, and the neural plate (NP) notochord (No), endoderm (A7.2, A7.1, B7.1, B7.2) and muscle (Mu) cells near the beginning of invagination (left) and near the end (right). There are two 3-D reconstructions associated with these images . The movies will open in a new window sized as indicated. View the pre-invagination movie [640x760 pixels, 4.1 MB] and the post-invagination movie [640x760 pixels, 3.8 MB].
The images above are 3-D projections of 110-cell embryos fixed during invagination and stained with phalloidin. The embryo on the right has invaginated further then the one of the left. What we see in both of these projections is that the endodermal nuclei have descended toward, the basal surface and radiating from these nuclei are actin-rich "caps". Double-staining with an antibody to microtubules showed that the actin caps colocalize with the microtubule organizing centers. Bundles of filaments within the actin caps also appear to extend, in most cells, all the way to the lateral cortex, which suggests that they could concievably mediate a cellular contraction during invagination.
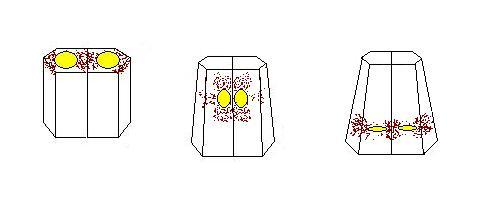
nuclear descent
Here is a schematic view of how nuclei migrate down in two cells in combination with the actin caps. The nuclei start off at the top of the apical surface with the actin caps radiating from the nuclei to the cortex. The nuclei rotate and dive down toward the basal surface of the cell and then later on they reorient. What could be happening as the nuclei move down is that the actin caps could be extending out toward the cortex and causing the cells to contract. If there were a sheet of these cells contracting, this could cause cells to fold inward. This idea of an actin cap contraction is only one possibility as to how a group of cells are forced to fold inward. We realize that there must be some force that pushes or pulls nuclei down and to push the close to each other, but we do not know what these forces are. Perhaps it could be that nuclei interact from cell to cell via contractile cables coupled by adhesive junctions, and thereby pull each other while squeezing the cells they inhabit like a toothpaste tube. The next page includes one preliminary finding on the behavior of isolated endoderm cells that supports this hypothesis.