Fixatives were used to preserve cellular structures at various stages of development. More specifically, embryos were fixed at the 64 cell stage before invagination and the 110 cell stage during and after invagination. We hoped to obtain snapshots of the distribution of cellular structures throughout the entire process of invagination.
Fluorescent dyes were used to stain specific preserved cellular structures. We used phalloidin to stain F-actin, propidium iodide to stain RNA/DNA, and tubulin antibodies (see below) to visualize microtubule organization in the cells.
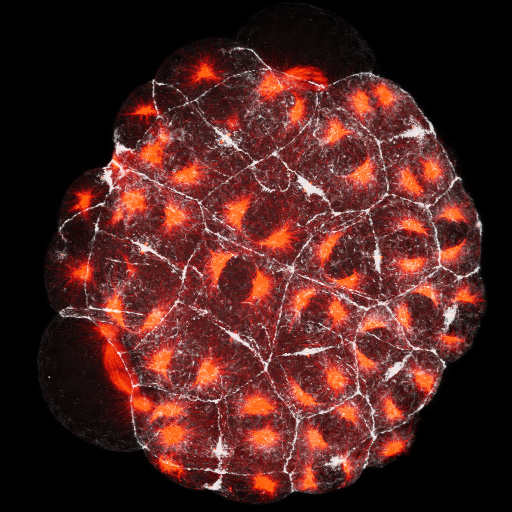
Boltenia stained for microtubules (red) and F-actin (white)

Using microneedles to isolate vegetal hemispheres
We performed micromanipulations with finely pulled glass needles. This allowed us to separate vegetal hemispheres from the animal hemisphere. In addition, endoderm blastomeres could also be isolated.
Time-Lapse microscopy was used to record movies of live embryos to enable us to see the dynamic process unfold. Confocal microscopy allowed us to see at high resolution the distribution of preserved cellular structures stained with fluorescent dyes.