During our exploration of gastrulation, we looked for evidence to support the different possible mechanisms by which the endoderm could invaginate.
Odell and colleagues explored a computer model for invagination in which there is assumed to be a contractile mesh of actin near the apex of the endoderm cells. Similar to a purse string tightening, the actin mesh is active and will contract the apices of the cells. It is assumed that the volume of the cells remains constant throughout the entire process of invagination. The constriction in the apices thus causes the basal ends of the cells to expand. this causes a fanning out of the sheet and induces invagination. In our fixed specimens, we looked for evidence of polarity in the concentration of actin towards the apical ends of the endoderm cells.
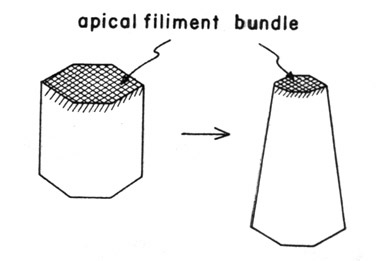
Contractile actin meshwork localized toward the apices of cells (figure from Odell et al 1981); contraction of the apical meshwork leads to cell shape change.
Odell and colleagues' "cortical tractor" model suggested there might be a flow of adhesive structures within the cell cortex. Adhesive proteins flow toward the apices and lateral borders of cells. This causes those ends of the cells to zipper up upon each other. Combined with apical contraction, this flow inevitably causes the cells to crawl up on themselves on the apices and roll up to drive invagination.
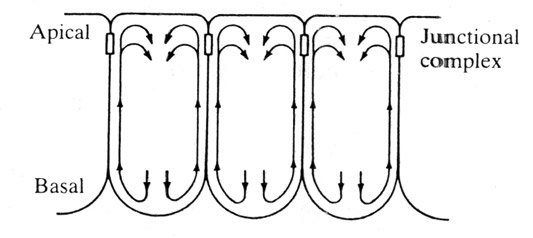
Cortical flow of adhesive structures (Jacobson et. al. 1986)
In this hypothesis, there is a difference between the adhesive properties of the apical sides and the basal sides of the invaginating cells. If the apices have a stronger adhesiveness for each other, then the stronger attraction near the apices causes the sheet to roll up and invaginate.
In this model, there are filopodial protrusions on the basal surfaces of the endoderm cells. These filopodia allow the basal ends of the cells to crawl out. If the volume of the cells remains constant, the basal lateral extension induces invagination of the sheet.
This hypothesis, which arose as a consequence of observations we made in the course of our project, suggests that cytoplasmic actin meshworks around the nuclei of the endoderm cells tend to want to contract as the nuclei descend during invagination. As the actin meshworks contract, they elongate the cell as they descend. This causes the sheet of cells to invaginate.
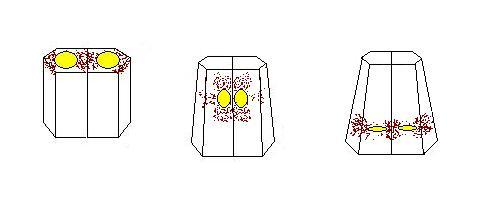
Nuclei pull together and descend with actin caps (Robinson et al 2003)