The dynamical process of invagination is shown in the following movie of a normal Boltenia embryo. [new window, 530x580 pixels, 3.8 MB]. This process involves the "diving in" of the endoderm cells during gastrulation. We explore how the hypothesized behavior of an apically biased constriction of the cortex and adhesion protein binding might cause an invagination in a sheet of cells.
We assert that the global process of invagination arises out of the local mechanical behavior of the endoderm cells. These cells know nothing about their neighbors, or where they lie in the invaginating sheet. Their behavior is governed entirely by Newton's laws of motion. The playing-out of these laws locally within each cell, and between cells, is what gives rise to the global phenomenon of invagination.
The descriptive and experimental data we obtained about the invagination process in ascidians embryos can be interpreted in many ways. However, just as there are many ways to imagine how this data might suggest a mechanism for invagination, the outcome of certain mechanical assumptions about cell shape change are very difficult to imagine. A computer model allows one to formulate specific mechanical assumptions about local cellular behavior and then to play these assumptions out, illuminating the consequences on a global scale.
DISCLAIMER: the model we developed during the course was largely conceived before we began empirical work, with the idea in mind that the model would make predictions about cellular behavior that we could look for empirically. Our empirical findings make us doubt that the model described here can provide a satisfactory mechanism for invagination in ascidians. However we intend to incorporate what we learned into future versions of the model using the same modeling framework.
The major force-generating and -transmitting components of the cell:
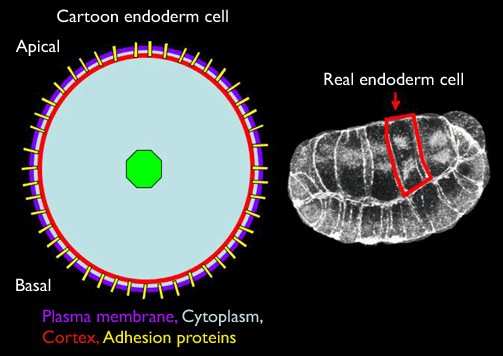
The diagram above represents the minimal essential features of real cells which we need to represent in a computer model: 1) the plasma membrane bounds the cell; 2) the cortex is composed of an actin-myosin meshwork that determines the mechanical force generating properties; 3) adhesion proteins pass through the plasma membrane and anchor in the cortex, and will also form non-covalent bonds with adhesion proteins on neighboring cells, thus allowing them to transmit forces to other cells; 4) the cytoplasm (structured by the cytoskeleton and nucleus) transmits forces within the cell.
How the cartoon version of the cell becomes a mechanical model of a cell:
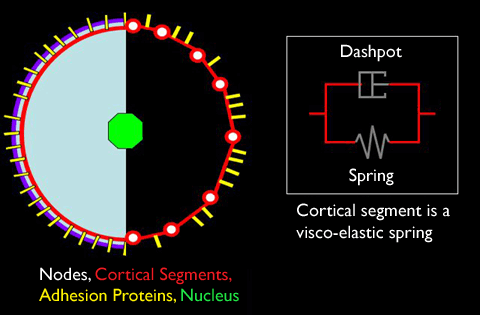
As diagrammed above, our model cell is constructed out of a bunch of discrete mechanical elements that have been chosen to represent the major components stated above. The cortex is built of many segments, each simulated by a visco-elastic spring. The elastic aspect of this spring allows it to be stretched and compressed, while the viscosity resists changes in the spring's length that happen quickly, but allow changes that occur slowly. This visco-elastic spring can also adopt different rest lengths depending on how it is being stretched or compressed. All of these visco-elastic springs are attached end-to-end such that they form a closed loop. These segments are populated by a number of adhesion proteins which can form non-covalent bonds with adhesion proteins from other cells; this bond creates an elastic force between them. The nodes are mathematical points at which the forces along an internodal cortical segment are summed. These nodes are also attached to the nucleus via struts (not shown above). As the struts and nucleus did not play major force-generating roles in our original hypothesis for ascidian invagination, it is sufficient to say that they act to transmit forces across the cell. (Note, however, that as a result of our experimental work we have come to suspect that the behavior of these components may be at least equally as important to invagination as the behavior of the cortex and cell-cell adhesions.)
First we consider the behavior of the model when the cortex is uniformly contractile. A single model cell is created with a non-circular shape. The visco-elastic springs that make up the cortex will try to contract to minimize the free energy of the cell. This causes the cell to round up:
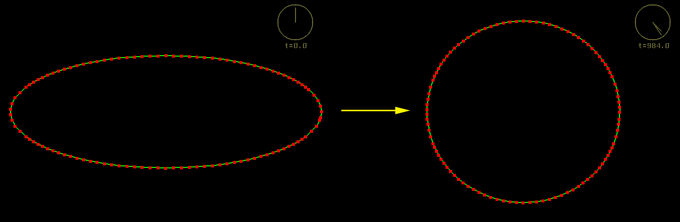
A single model cell rounding up because of uniform cortical contractility (courtesy of Ed Munro). Click the image to see the movie. [new window, 780x700 pixels, 464 KB].
Next consider the behavior of the model when the cortex is uniformly contractile and adhesion proteins are added:
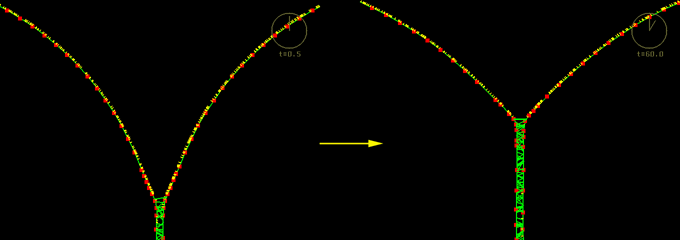
Two model cells zipping together and causing a cortical flow (courtesy of Ed Munro). This image is associated with a low resolution movie [new window, 680x710 pixels, 444 KB]. and a high resolution movie [new window, 680x710 pixels, 1.3 MB]. Click the image to see the low resolution movie.
As the distance between adhesion proteins decreases, the probability that they will bump into each other and form a bond increases. Two cells are brought near each other and their adhesion proteins cause the cells to zipper up. Immediately we note that the nodes are moving into the lateral zone. Thus the zipping-up creates a flow of the cortex into the lateral zone. As one linker binds to another, it applies a force on the segment of the cortex which causes it to stretch, which ends up pulling another segment of the cortex closer. As the cortex is populated with many adhesion proteins, it means that the next adhesion protein becomes closer and therefore has a greater probability of binding than before. If the adhesion proteins are sufficiently dense along a segment, the binding of one of the proteins will cause the next protein to be pulled close enough to bind, and so on. This will create a constant pulling on the cortex, which is what creates the flow and the appearance of the zippering up. The cells do not zipper up completely. The length of the lateral zone reaches an equilibrium when the force it takes to stretch the cortex further cannot be overcome by the elastic force of adhesion proteins binding.
We now add a biased apical constriction of the cortex:
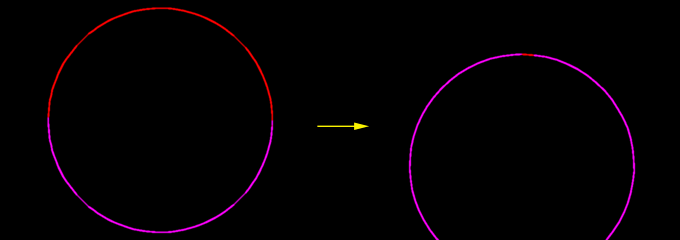
A model cell with stronger contraction on the apical side; the cortical segments initially designated as "apical" are colored red. This image is associated with a low resolution movie [new window, 680x710 pixels, 52 KB]. and a high resolution movie [new window, 680x710 pixels, 112 KB]. Click the image to see the low resolution movie.
Here, in a single model cell, we see the consequences when the visco-elastic springs on the apical side can contract stronger than those on the basal side. In this tug-of-war the apical side will always win, and so the segments will be stretched apically. Not surprisingly this creates the flow towards the apical side. When too much cortex accumulates on the apical side of the cell, which is simulated by a certain decrease in the internodal segment length, the nodes will be deleted. When the internodal basal segments are stretched too far, which is analogous to a large decrease in the density of the cortex, a node will be added. This enables the model to simulate the real processes that remove and insert cortex and plasma membrane.
In the next sequence there is a biased apical constriction of the cortex and adhesion protein binding between neighboring cells:
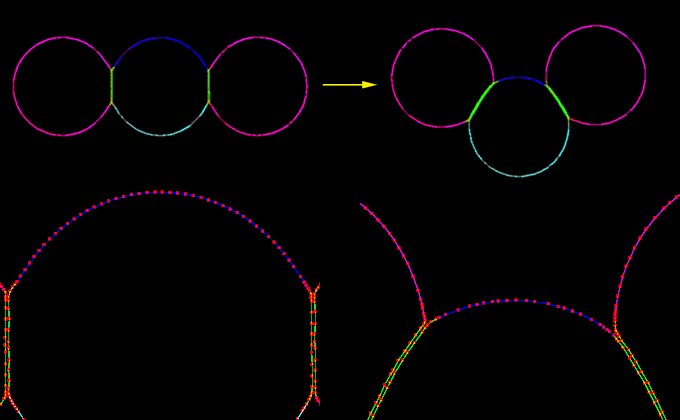
An apically-biased contracting model cell, sandwiched between two non-contractile neighbors, invaginates. This image is associated with a low resolution movie [new window, 680x710 pixels, 1.8 MB]. and a high resolution movie [new window, 680x710 pixels, 4.1 MB]. Click the image to see the low resolution movie.
Three cells are shown. The middle cell has the apical-biased contractile cortex. The two end cells do not have a contractile cortex. All three cells are populated by adhesion proteins. The middle cell performs a kind of invagination. The biased apical contraction causes the cortex of the middle cell to flow towards the apical side. As the adhesion proteins between the cells begin to bind, the zippering-up process occurs. The most apical adhesion proteins that are bound will transmit the force of the middle cell's flow of the cortex to the cortex of the neighboring cell. The fact that there is still a flow of the cortex in the middle cell means that the adhesion proteins that bind and act to transmit the contractile force to the neighboring cell, only are bound for a short while. The continual binding of new adhesion proteins will keep transmitting some of the contractile force to the neighbor cells' cortex, which will allow the invagination to occur.
As in the previous section, here we show the behavior of the model when there is a biased apical constriction of the cortex and adhesion protein binding between neighboring cells. However, now we have a small sheet of apically-biased contractile cells:
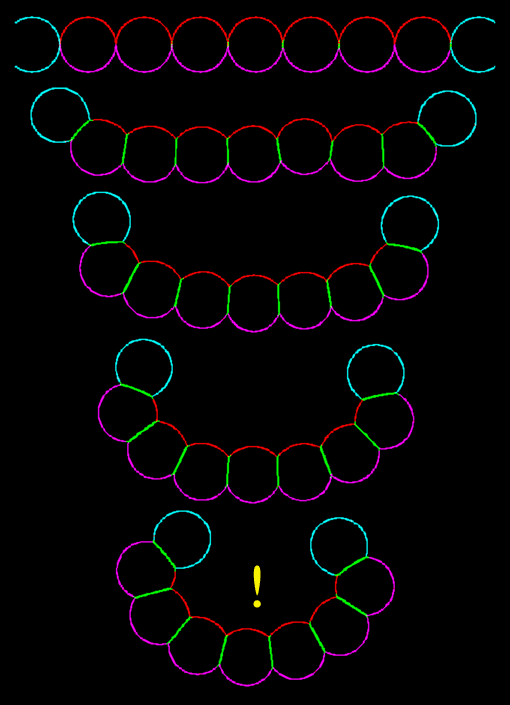
A sheet of apically-biased contracting model cells, with non-contractile neighbors at the edges, invaginates very nicely indeed! Click the image to see the movie [new window, 680x710 pixels, 560 KB].
The cells invaginate! This shows that the hypothesized behavior of a biased apical contraction coupled with adhesion protein binding is sufficient to create an invagination in cells. Restated, the hypothesized local mechanical behaviors were successful in producing the global pattern of invagination.