Asymmetric cell division is one of the fundamental mechanisms for making embryonic cells different. Different embryos work many variations of this common scheme, but the basic idea is always the same: A mother cell segregates cell fate determinants in response to a spatial cue to establish a specific axis of polarity; the mitotic spindle aligns itself along this axis and positions a cleavage furrow such that the two daughter cells inherit a full set of chromosomes and different cell fate determinants. As we learn more about how asymmetric cell division works in different contexts, it becomes clear that much of the underlying machinery is organized into conserved networks of gene products whose interactions govern the establishment and maintenance of cortical and cytoplasmic polarities, positioning of the mitotic spindle, and positioning and assembly of the contractile ring. The hope is that we can begin to understand asymmetric cell divisions in terms of the functional dynamics of these cytomechanical modules interacting with one another in specific ways.
Our efforts to tackle these problems focus on cell polarization and asymmetric cell division in the C. elegans zygote, using a combination of experimental and computational approaches including:
Polarity is established in the C. elegans zygote in response to a localized cue associated with the sperm MTOC that forms near the site of sperm entry. During the first mitotic interphase, this cue triggers a rapid segregation of cortical and cytoplasmic determinants. The "anterior" Par proteins, Par-3/Par-6/Pkc-3, become enriched on the cortex opposite the sperm MTOC, while "posterior" Par proteins Par-1 and Par-2 accumulate in a complementary posterior cortical domain. Distinct mechanisms maintain these asymmetries during M-phase as the centrosomes and associated pronuclei migrate towards the center of the zygote to assemble the first mitotic spindle. During anaphase, the mitotic spindle signals to the cortex to position an actomyosin-based contractile ring that pinches the zygote in two to produce two daughters with distinct developmental potentials.
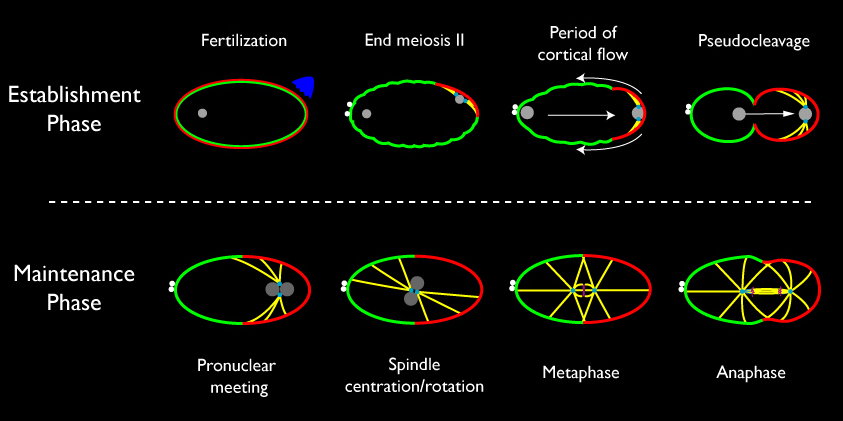
Schematic view of events that lead up to the first asymmetric division of the C. elegans zygote. Nuclei are gray; Microtubules are yellow; Chromosomes are magenta; small filled cyan circles represent centrosomes; small filled white circles are polar bodies extruded during meiosis; red and green along the cell cortex represent cortical domains enriched in anterior PAR proteins (green = PAR-3, PAR-6, PKC-3) and posterior ( red = PAR-1 and PAR-2) PAR proteins.
In the past few years, work from our group and others has produced to the outlines of a working hypothesis for polarity establishment. Before and during polarization, transient focal contractions of a cortical actomyosin network put the entire cortex under tension. Local inhibition of focal contraction near the sperm MTOC causes the remaining network to contract away from the sperm MTOC, driving a cortical flow of F-actin, myosin, and Par-3/Par-6/Pkc-3 towards the opposite end of the zygote. This contraction stalls halfway across the zygote, resulting in the formation of a stable anterior cap enriched in myosin II, Par-3/Par-6/Pkc-3, and other cortical proteins. The anterior restriction of Par-3/Par-6/PKC-3 is followed by the rapid accumulation of Par-1 and Par-2 in a complementary posterior domain, consistent with the idea that Par-3/Par-6/Pkc-3 inhibit cortical association of Par-1 and Par-2, possibly through the ability of Pkc-3 to phosphorylate these proteins (e.g. Hao et al, 2006).
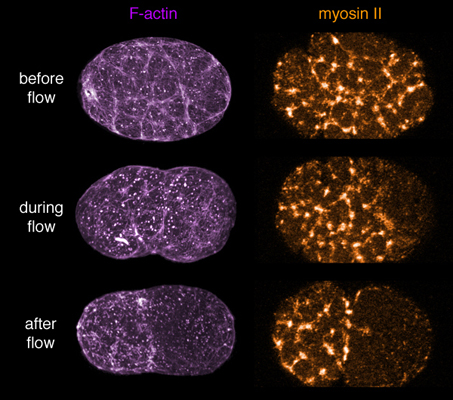
F-actin and myosin coorganize a contractile network that "caps" to the anterior pole during polarity establishment. Click on the image to see a quick time movie
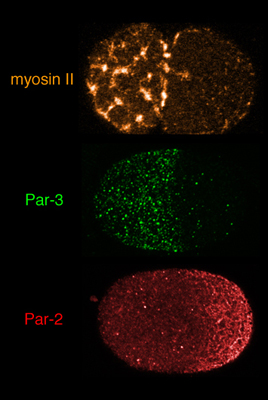
Redistribution of myosin II, Par-3/Par-6/Pkc-3 and Par-2 during polarity establishment. Click on the image to see a quick time movie
Subsequent work by many groups (Jenkins et al. 2006; Motegi and Sugimoto 2006; Schonegg and Hyman 2006; Schoff et al, in prep) has shown: (a) that cortical contractility during establishment is controlled by the small GTPase Rho-1; (b) that the sperm cue is mediated through local down-regulation of Rho activity through inhibition of the Rho-GEF ECT-2 and/or activation of the Rho-GAP Cyk-4, and (c) that Rho-1, or factors that regulate its local activity, are themselves transported by cortical flows. In other experiments (Munro et al 2004, and unpublished data), we have found that many of the Par proteins also modulate cortical actomyosin dynamics and flow to affect their own redistribution. These and other observations identify the core structure of a cytomechanical module that transforms a transient spatial cue into a functionally polarized cortex and cytoplasm.
The pictures below link to QuickTime movies. The movies open in a new window sized as indicated.
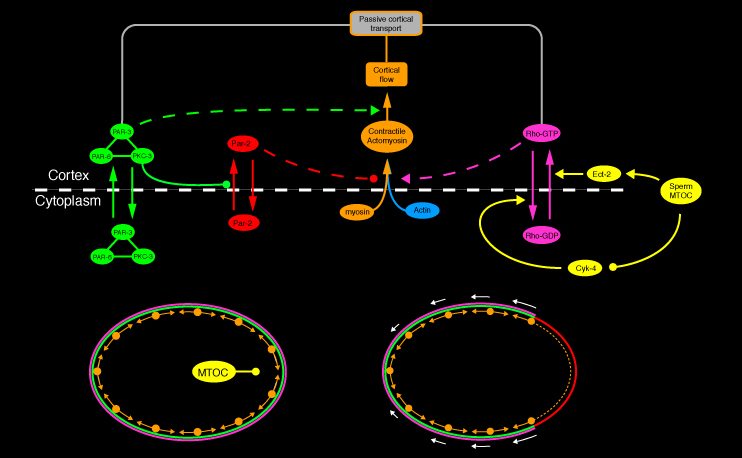
A working hypothesis for cortical polarity establishment. Rho-dependent actomyosin contractility puts cortex under tension. Local downregulation of Rho triggers asymmetrical contraction and cortical flows that concentrate Par-3/Par-6/Pkc-3 in the anterior, allowing Par-1 and Par-2 to associate with the posterior cortex. The Par proteins in turn feed back to regulate actomyosin contractility and thus their own transport.
To explore how a robust polarization mechanism might emerge from this network of interactions, we have developed a computational approach that explicitly captures the local interplay between biochemistry and mechanics. We model the cortex (in 2D) as a connected belt of linear segments, each representing a small patch of contractile cortex, enclosing a single cytoplasmic compartment. We endow each cortical segment with active contractile and passive viscoelastic properties that we hypothesize to emerge from local interactions among motors, filaments, and cross-linkers within the cortical patch. Cortical segments exchange forces with one another, with the incompressible cytoplasm inside, and with a rigid elliptical “eggshell” outside. We resolve these forces according to Newton’s Laws to compute how the segments deform and move. At the same time, each element acts as a chemical compartment, housing specific factors whose local densities change either through local kinetics (assembly/disassembly, recruitment, complex formation, enzymatic transformation), exchange with other compartments, or because they are concentrated, rarified or moved as the finite element compartments that contain them deform and move. Finally, we make the constitutive mechanical properties of each compartment depend on the densities of F-actin, cross-linkers, myosin, etc, or their assembly and disassembly kinetics.
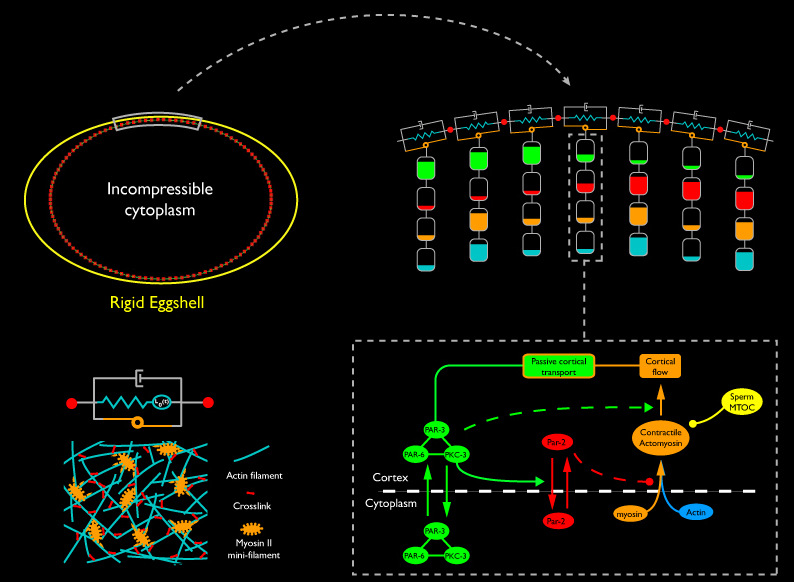
Analysis of this model (Munro and Hechter, in prep) shows that the focal contractility cycle we observe during polarity establishment could emerge as a robust consequence of the basic mechanochemistry of cross-linked actomyosin networks. The same dynamics that explain the local contractility cycle can also explain how the cortex can produce a self-limiting global contraction to form a stable anterior cap in response to a local weakening by the sperm cue. Finally, we have found that segregation of Par-3/Par-6/Pkc-3 to the anterior during polarity establishment cannot result from simple reversible association of a trimeric complex with a moving cortical meshwork. Additional experiments, plus recently published data from other groups, lead us to a new hypothesis: that Par-3, Par-6 and Pkc-3 participate in a spatially distributed oligomeric network, which is remodeled, and whose components are redistributed, through local reversible association with the actomyosin cortex. We are currently exploring this hypothesis using a combination of experiments and computer simulations (see below).
The pictures below link to QuickTime movies. The movies open in a new window sized as indicated.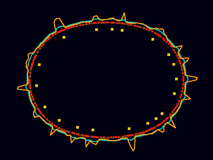
The model cortex robustly reproduces the wild-type focal contractility cycle.
[640 x 480 pixels, 12 MB]
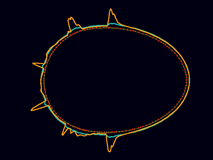
The same model responds to local weakening with a self-limiting global contraction to form a stable anterior cap.
[640 x 480 pixels, 3.3 MB]
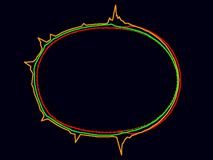
If Par-3, Par-6 and Pkc-3 form a coherent cortical network, then the Par proteins segregate correctly during cortical flow.
[640 x 480 pixels, 6.7 MB]
In other work, we are exploring how the zygote stabilizes cortical asymmetries during maintenance after the initial cue has departed. CCD postdoc Adriana Dawes has found that a conserved network of cross-regulatory interactions among the Par proteins can implement a bistable switch mechanism that could help “lock in” distributions of Par proteins established by cortical flow, and she is now testing this idea experimentally. In other work, we have found that polarity maintenance, CDC-42 acts through distinct effectors within at least three distinct feedback loops to stabilize cortical polarities. First, CDC-42 activates myosin contractility through a conserved kinase to transport CDC-42 itself, or factors (GEFX in the diagram below) that regulate its activity. Second, CDC-42 binds and promotes cortical association of PAR-6/PKC-3 (Aceto et al, 2006). PAR-6/PKC-3 in turn inhibits cortical association of PAR-2 (Gou et al, 2006) to relieve its inhibition of cortical myosin II recruitment (Munro et al, 2004, Schoff et al, in prep).
These two positive feedback loops maintain a persistent contractile asymmetry, without which Par-3/Par-6/Pkc-3 gradually spread back towards the posterior. We have found that the same feedback loops can also “rescue” cortical polarity during maintenance by amplifying a very weak asymmetry if the normal establishment mechanism fails. However, computer simulations predict that this positive feedback loop, acting alone, would lead to a runaway contraction of the anterior cap (see Maintenance Movie 1 below). And indeed, we have identified a second negative feedback loop in which Arp2/3 acts downstream of Cdc-42 to promote local assembly of branched F-actin within the anterior cap, which mechanically resists, or otherwise inhibits, myosin contractility. Computer simulations predict that this combination of positive and negative feedback loops can lead to a dynamical balance of mechanical forces that could stabilize cortical asymmetries in the absence of an external cue (see Maintenance Movie 2 below).
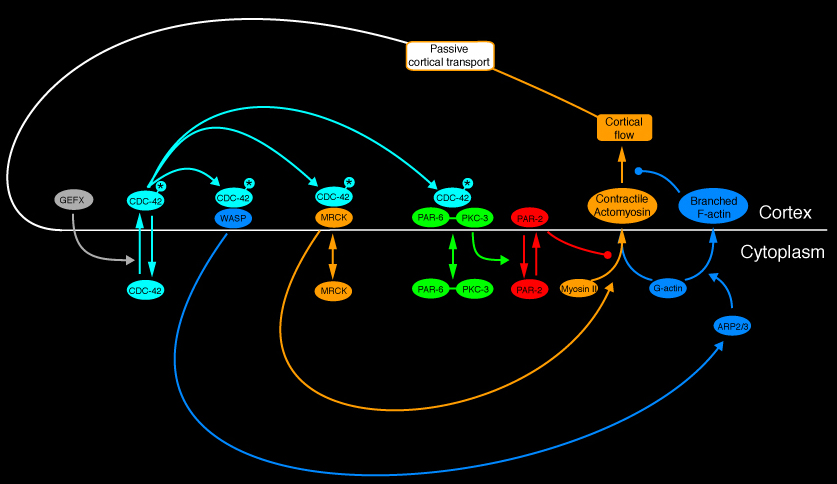
A network of positive and negative feedback loops that might stabilize cortical asymmetries during polarity maintenace.
Maintenance movie 1:
Positive feedback loops acting alone lead to a runaway contraction of the anterior cap.
[640 x 480 pixels, 2.2 MB]
Maintenance movie 2:
A combination of positive and negative feedback loops acting together lead to a dynamic stabilization of the anterior cap and the pattern of cortical flow seen in wild type embryos during maintenance.
[640 x 480 pixels, 2.3 MB]
We see the C. elegans cortex is a very general model for exploring how actomyosin contractility works in non-muscle cells. How do mesoscale viscoelasticity and contractility emerge from local filament assembly dynamics, cross-linking and myosin motor activity? During the first cell cycle, in the span of just 15 minutes, the zygote somehow “tunes” these local properties to reconfigure global contractility to do at least three distinct jobs - first to establish polarity in response to a transient localized cue, then to maintain polarity in the absence of a cue, then to respond to signals from the mitotic apparatus to assemble and constrict a contractile ring. How does this work? To address these questions, we are pursuing three complementary approaches.
Our previous work (described above) suggests that the cortical segregation of Par proteins during polarity establishment cannot be explained by reversible association of a trimeric PAR-3/PAR-6/PKC-3 complex with a moving actomyosin network. We are currently exploring alternative hypotheses, focusing on the following questions: How do the Par proteins interact with one another and with the actomyosin cytoskeleton to maintain a stable enrichment at the cortex? How do the Par proteins and cortical actomyosin exchange forces? To what extent do the Par proteins contribute mechanically to forming and/or stabilizing the cortical domains they inhabit? Again, we are addressing these questions using a combination of quantitative imaging, molecular genetic approaches, and detailed agent-based models.
We have found that mutating or depleting any of the PAR and MEX proteins, either singly or in combination, leads to distinct and often profound defects in cortical contractility and flow during polarization. This suggests that PAR and MEX proteins feedback in multiple ways to modulate cortical actomyosin cytoskeleton. We are using approaches outlined above to characterize the organization and dynamics of the actomyosin cortex in embryos mutant for or depleted of specific Par and Mex gene products to determine the nature of these modulatory effects and then incorporating the results into our computational models.
We have also begun to explore the mechanisms that govern cytokinesis in the C. elegans embryo. The basic idea is to complement our comparative studies of cytokinesis and cytokinesis-related phenomena in Drosophila and marine vertebrates in a model organism that is readily amenable to both imaging approaches and molecular/genetic perturbation. We are principally interested in three questions: 1) How does the mitotic apparatus read previously established polarity cues off the cortex to position an asymmetric mitotic spindle? 2) How does the mitotic spindle talk back to the cortex a short time later to tell it where to position a contractile ring? 3) How does the cell rapidly assemble and then dissipate a local contractile structure?
As with our studies on the establishment and maintenance of polarity, we combine experiment and computation: That is we combine live imaging and 3D reconstructions of fixed embryos, molecular, genetic and pharmacological perturbations of the cell division apparatus using standard tools, and detailed computational models that incorporate known or suspected properties of and interactions among key molecular players in cytokinesis.