Ascidians offer a surprisingly untapped system with a unique suite of advantages for morphogenetic studies. Basal members of the chordate lineage, they perform simple versions of all the classic vertebrate morphogenetic behaviors - endoderm invagination, neural tube closure and elongation, notochord convergent extension etc. Invagination, in which a sheet of cells coordinately deforms to form a hollow tube, has been studied in a diverse array of organisms and developmental contexts, from gastrulation in sea urchins (Davidson 1995) and Drosophila (Leptin & Grunewald 1990, Sweeton et al. 1991), to neurulation in amphibians, birds, and mammals (Schoenwolf & Smith 1990), and during later development in events such as eye and trachea formation. One of the main impediments to establishing the mechanics of invagination has been the technical difficulties inherent in visualizing cell shape changes at sufficiently high spatial and temporal resolution, in live embryos. Most invaginations occur with hundreds to thousands of cells, in embryos that are often opaque, muti-layered, too large to view at high magnification intact, or not amenable to manipulations such as explanting and ablations.
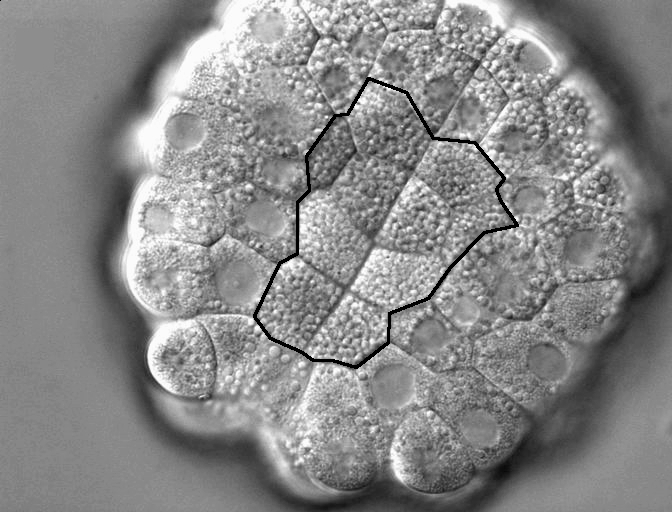
Figure 1. DIC timelapse invaginating Corella inflata embryo, in vegetal view. Ten endoderm cells which will invaginate outlined in black.
In contrast, the invagination at the start of ascidian gastrulation involves just 10 endoderm cells (each the size of a C. elegans embryo) in a 150 um embryo of c. 100 cells (Fig. 1); a few cell cycles later, a sheet of 80 cells neurulates and a mere 40 notochord cells converge and extend (Munro & Odell 2002, Munro et al. 2006). In all these cases, large cell size makes it possible to characterize the underlying cytoskeletal and adhesive dynamics at high resolution and to link individual cell shape changes unambiguously to global deformations of the embryo, while the low cell count facilitates making detailed computer models of the process. Several species of ascidian, such as Corella inflata, are transparent, making it possible to observe cell dynamics at high resolution with time lapse microscopy movie. Early cell fate specification, cell-autonomous morphogenetic behavior of explants, and the tolerance of intact embryos and explants to culture in seawater, make it possible to distinguish the contributions of cell-intrinsic behaviors from those that require adhesive coupling or other mechanical or biochemical interaction with neighbors. Three species’ genomes have been sequenced, facilitating the design of fluorescent probes and morpholino constructs, and these can be expressed or introduced by electroporation and/or microinjection. Because the ascidian genome is not duplicated, revealing the molecular underpinnings of specific behaviors is often simpler than in higher chordates. Thus, we can exploit the known genome sequence, the single-copy status of many genes, the early establishment of lineage-specific gene expression, and morpholino-based gene product knockdown screens to identify the proteins implicated in particular aspects of a morphogenetic mechanisms. Our work to date focuses on endoderm invagination, but also positions us to study other classical morphogenetic events, such as neurulation, in these model chordate embryos.
We have completed a detailed kinematic description of endoderm invagination using timelapse DIC of intact and half embryos and explants of one to several cells, and confocalling of fixed embryos stained with phalloidin and antibodies to phospho-myosin and tubulin. We find that, similar to Drosophila ventral furrow ingression, ascidians invaginate their endoderm in two steps, the first characterized by apical constriction and the second by apicobasal shortening. Actively contractile myosin, comprising the two phosphorylated forms Pser19 myosin regulatory light chain (MRLC) and Pser19/thr18 MRLC, is appropriately localized to drive each step. The apical constriction, but not basolateral shortening, requires rho-Kinase signaling. We are currently completing a manuscript on this work with François Robin and Patrick Lemaire in Marseilles, France, who have obtained complementary kinematic and regulatory results using 4D confocal microscopy of live specimens and a dominant negative RhoA construct.
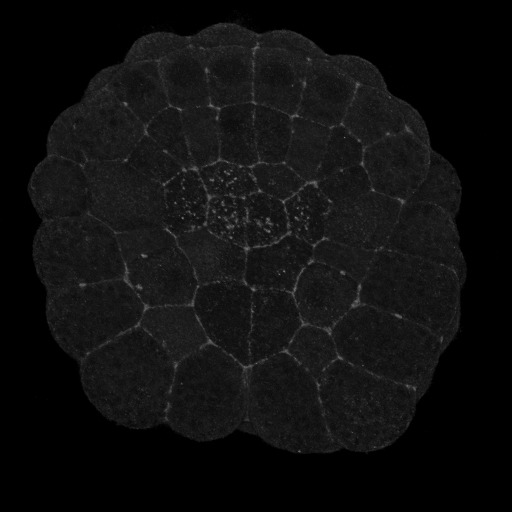
|
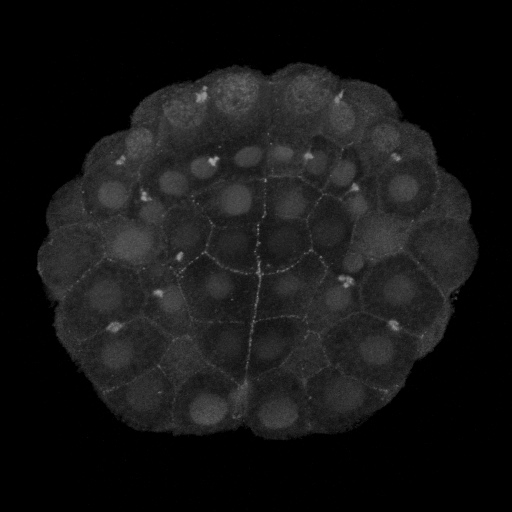
|
Figure 2. Vegetal views (anterior up) of invaginating ascidian embryos. A) Pser19-MRLC stains circumpical of most cells and apical of the endoderm cells, particularly the six anterior endoderm. B) Pser19/thr18 stains the circumapical of all and only endoderm cells.
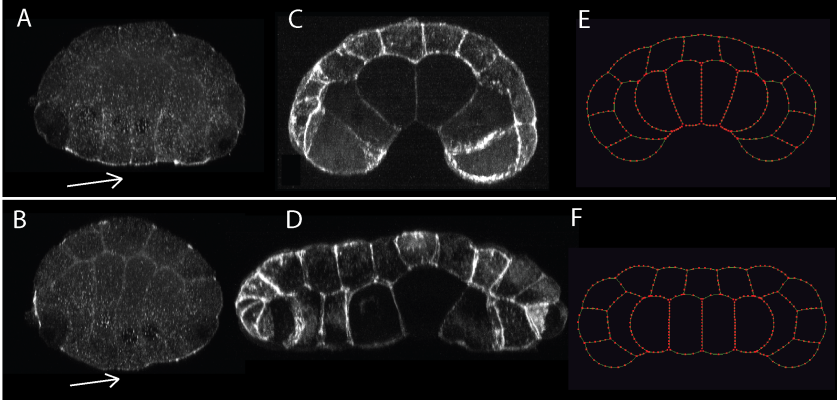
Figure 3. We saw elevated ser-19-phosphorylated myosin regulatory light chain on the endoderm apical surface (arrow) of control embryos in early gastrulation, shown in cross-section, vegetal side down. (A), contributing to invagination (B); the rho-kinase inhibitor Y-27632 greatly reduced this localization (arrow) (C), and prevented invagination (D). Computational simulations assuming strong apical contractility mimic the controls (E), and simulations with reduced apical contractility mimic Y-27632 treated embryos (F).
To complement our experimental work Munro and Sherrard have created a computational simulation of morphogenesis in two dimensions, representing an entire cross-section of the embryo. This model is a direct outgrowth of Munro’s model of cell motility and convergent extension. Previous models of tissue morphogenesis (e.g., Odell et al. 1981, Jacobsen et al. 1986, Hardin & Keller 1988, Clausi & Brodland 1993, Davidson 1995), though providing crucial tests of mechanical plausibility, have relied largely on continuum representations of cell mechanics and lack explicit reference to the underlying molecular machinery. We have developed a hybrid approach that combines a compartmental representation of the cell cortex with an agent-based model of cadherin-mediated adhesion (Fig. 4).
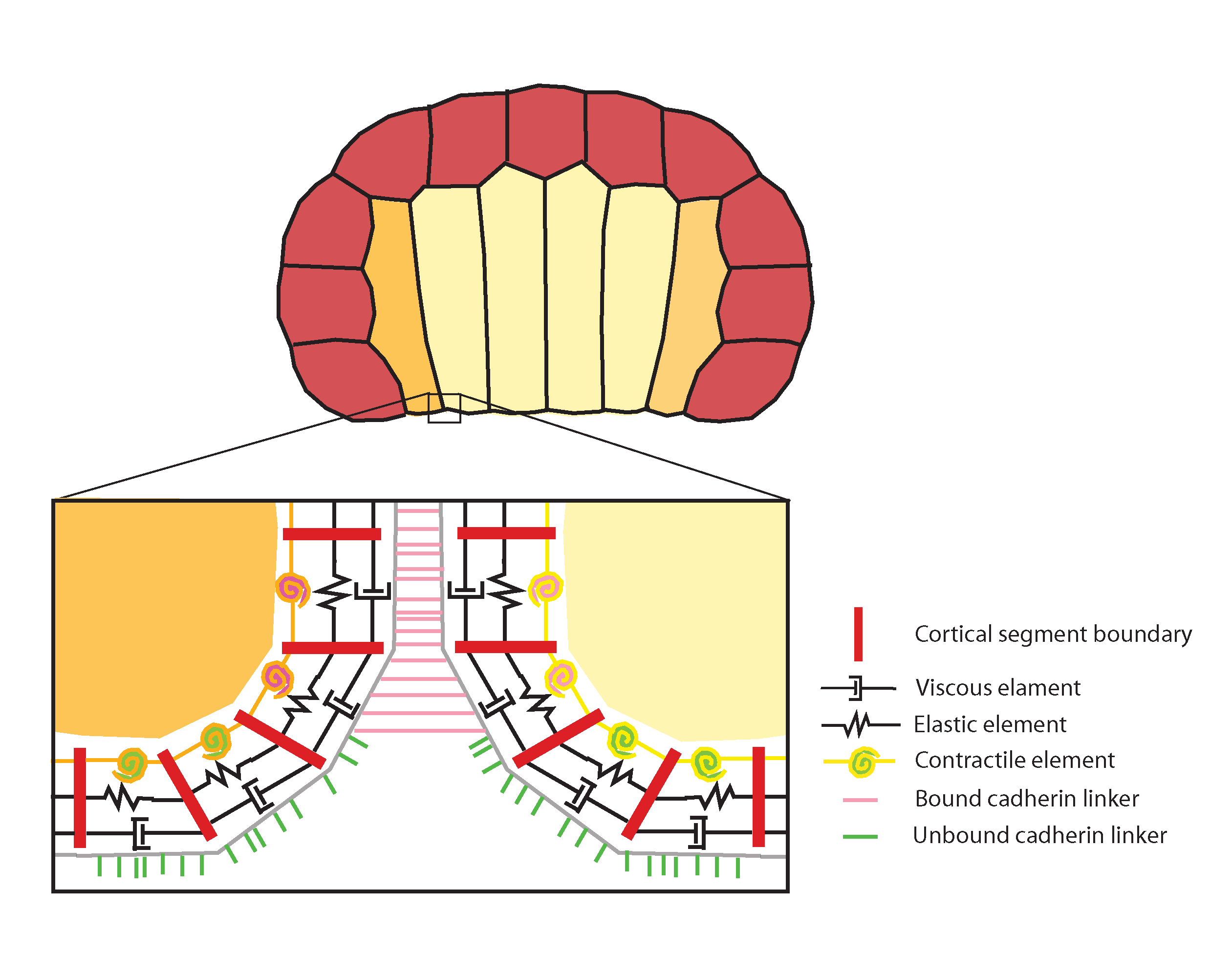
Figure 4. Computational model of invagination, representing two-dimensional cross-section of ascidian embryo (endoderm yellow, mesoderm orange, ectoderm red). Detail: the cortex is represented as a belt of viscous (dashpot symbols), elastic (zigzag symbols) and contractile elements and linkers (bound: pink; unbound: green) that bind and unbind from the cortex and each other stochastically and are transported by cortical flow. Different cell types can be endowed with distinct contractile, viscous, and elastic properties to mimic experimental observations (e.g., higher apical contractility on endoderm cells) or to explore the consequences of hypothetical manipulations. In addition, empirical observations, such as a feedback of contractility being downregulated in adhesively bound (i.e. basolateral) regions, can be built into the simulation. See also morphogenesis tutorial.
Two features distinguish our approach from all previous efforts: The first is a close correspondence between model building blocks and empirical observables at molecular and subcellular levels. The second is the complete absence of any top-down constraints on cell geometry or tissue organization. In our model, cell shapes, organization, and neighbor exchanges emerge solely from the local rules governing individual cortex parts. We can represent hypothetical local elevations in cortical tension, to correspond to data from kinematics and immunofluorescence observations of myosin activation, and test whether our interpretations of invagination mechanism are feasible. We can also take a bottom-up approach, performing systematic searches of parameter space (using a simplified version of the model that subsumes adhesion kinetics into tension) to discover, for a given starting geometry, the range of relative tension values in different cell types and locales that is capable of generating invaginations.
Clausi, D.A. and G.W. Brodland, 1993. Mechanical evaluation of theories of neurulation using computer simulations. Development 118: 1013-1023
Davidson, L., G.F. Oster, R. Keller, and M.A.R. Koehl, 1995. Measurements of mechanical properties of the blastula wall reveal which hypothesized mechanisms of primary invagination are physically plausible in the sea urchin Strongylocentrotus purpuratus. Developmental Biology 209: 221-238
Hardin, J. and R. Keller, 1988. The behaviour and function of bottle cells during gastrulation of Xenopus laevis. Development 103(1): 211-230.
Jacobsen, A.G., G.F. Oster, G.M. Odell, and L.Y. Cheng, 1986. Neurulation and the cortical tractor model for epithelial folding. Journal of embryology and experimental morphology 96: 19-49.
Keller, R., L. Davidson, and D. Shook, 2003. How we are shaped: The biomechanics of gastrulation. Differentiation 71: 171-205.
Leptin M. and B. Grunewald, 1990. Cell shape changes during gastrulation in Drosophila. Development 110: 73-84.
Odell, G.M., G.F. Oster, P. Alberch, B. Burnside, 1981. The mechanical basis of morphogenesis I. Epithelial folding and invagination. Developmental Biology 85: 446-462.
Schoenwolf, G.C. and J.L. Smith, 1990. Mechanisms of neurulation: traditional viewpoint and recent advances. Development 109: 243-270.
Sweeton, D., S. Parks, M. Costa, E. Wieschaus, 1991. Gastrulation in Drosophila: the formation of the ventral furrow and posterior midgut invaginations. Development 112: 775-789.