George von Dassow and Victoria Foe
The mechanisms by which cells initiate cytokinesis remain poorly-understood. Unsolved questions include: how the mitotic apparatus communicates its position to the cell surface; what pre-existing structure, if any, assembles into the contractile ring; and what determines the time of furrowing? Historically, biologists have sought to reconcile conflicting observations on diverse cells into a unified explanation of animal cell cytokinesis. Even so neither of the two popular textbook stereotypes - polar relaxation or equatorial stimulation - matches more than half the known facts about the process. Indeed, even isolated cells are so diverse in form, size, timing, mechanics, etc. that any one-size-fits-all mechanism strains credibility. There is a wealth of variation in cytokinesis among animal embryos; some of these natural experiments are shown in Figure 1. Recent experiments confirm that cells differ in fundamental aspects of cytokinesis; for example, while Rappaport's experiments with sand dollar eggs showed that juxtaposition of two mitotic asters suffices to induce a furrow, recent results suggest that spindle mid-zone microtubules stimulate furrowing in mammalian cells, and new observations on frog eggs suggest a role for cortical, i.e. non-spindle and non-astral, microtubules.
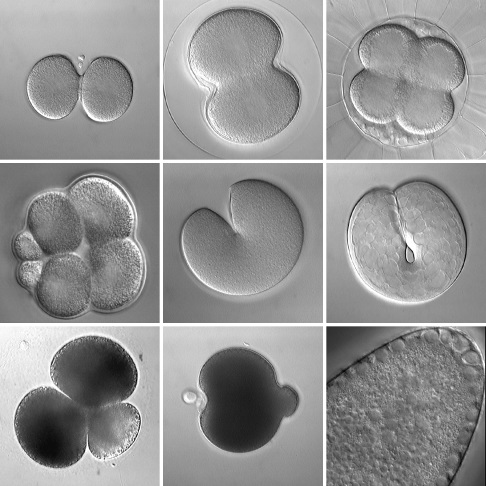
Figure 1: Various embryos during early cleavage. Top row: normal cytokinesis in the nemertean Cerebratulus (left), the urchin S. droebachiensis (middle), and the ascidian Corella (right). Middle row: variations on cytokinesis include unequal cleavage in urchin embryos (left; the urchin S. purpuratus), and "unipolar" cytokinesis in the embryonic cells of cnidarians (middle; the jellyfish Aequorea) and ctenophores (right; Pleurobrachia). Bottom row: cytokinesis-like processes include polar lobe formation in diverse spiralians including scaphopods (left; Pulsellum) and bivalves (middle; the clam Acila), and somatic budding (right; the wasp Nasonia). All panels are DIC images taken from time-lapse movies.
We got interested in cytokinesis during studies on two aspects of syncytial development in Drosophila. One of us studied nuclear migration during early mitoses, finding that astral microtubules locally re-organize a cytoplasmic actin network; this local change stimulates bulk cytoplasmic streaming until nuclei achieve a uniform distribution. The other studied bud formation at a slightly later stage, after nuclei arrive at the cortex of the syncytium but prior to cellularization, and concluded that pseudo-cleavage furrows arise from directional movement of cortical actin and myosin II along astral microtubules (for 3-D reconstructions describing the cytoskeleton during bud formation, visit DownTheTubes). We wondered whether these two phenomena, which otherwise seem like oddities of syncytial development, might share homologies to classical cytokinesis (see Foe et al. 2000).
We are examining the configuration of microtubules, F-actin, and myosin during cyokinesis in early sea urchin embryos, and find much in common despite the very different geometrical context. Early urchin embryos, like early fly embryos, make an extensive cytoplasmic actin network during mitosis, which all but disappears by the end of cleavage (Fig. 2). The actin network appears to be re-organized by astral microtubules. As in fly embryos, myosin II disappears from the cortex and then is recruited to the furrow region in phase with the cell cycle. We are also investigating the unequal fourth cleavage in urchin embryos. At the end of third cleavage, the vegetal cortex apparently captures one of the two centrosomes, leading to spindle displacement and re-orientation (Fig. 3). The cortically-located spindle pole forms a flattened aster, at the edge of which the cleavage furrow forms. This is reminiscent of pseudocleavage furrowing in Drosophila, except that in this case complete cleavage results.
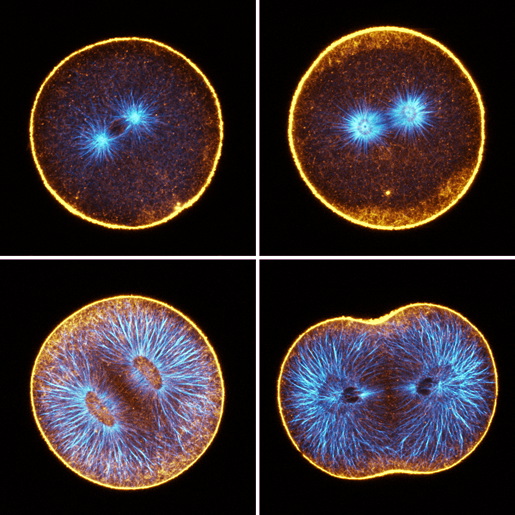
Figure 2: Confocal sections of sand dollar embryos in first cleavage; orange=F-actin, blue=microtubules. Top left: prophase; during interphase and prophase, we detect very little F-actin in the deep cytoplasm. Top right: metaphase; cytoplasmic F-actin becomes apparent in metaphase. Sometimes, as shown here, the deep actin network first appears as an annulus around the cleavage plane. Bottom left: mid-anaphase; the deep actin network is most prominent during anaphase, but the density of F-actin is roughly complementary to the density of microtubules. Bottom right: telophase; the deep actin network disappears progressively during cleavage, and remains longest in the furrow zone.
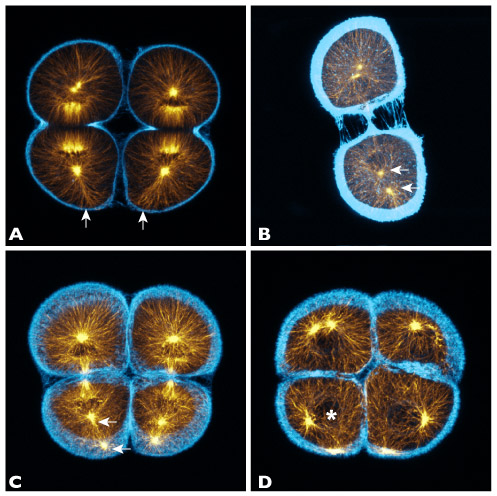
Figure 3: Projections of 10-20 confocal sections of purple urchin embryos near the end of third cleavage; orange=microtubules, blue=F-actin. Top left: telophase; as the cells cleave, we observe a loose bundle of microtubules, prominent against the rest of the aster, that reaches the vegetal cortex (arrows). Top right: late telophase; one of the two nascent MTOCs (arrows) in the vegetal daughter becomes displaced away from the nucleus. Bottom left: early interphase; the displaced MTOC migrates to the vegetal cortex, while the other remains associated with the re-forming nucleus. Bottom right: interphase; once the nucleus reforms near the cell center (asterisk), it subsequently migrates into position between the two MTOCs.
Our goal is to characterize a tool-kit of homologous processes which all cells likely share, and investigate how those cytomechanical modules have been deployed and weighted differently to accomplish diverse modes of cell cleavage and cleavage-like phenomena. That is, rather than pursuing the One True Mechanism, we suspect it will be more realistic to explain cytokinesis in terms of conserved sub-processes with different contributions in different contexts.
cytomechanical modules 2003 • back