(Sagar D. Joshi)
Morphogenesis involves many processes—invagination, evagination, ingression and involution etc., where cell shapes change have long lasting consequences for embryonic development. Invagination is the morphogenetic process by which a cell sheet bends to form a pit or groove, as observed during drosophila and chick embryo development (figure 1).
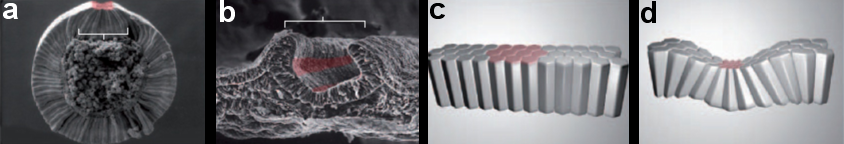
Figure 1: Cellular basis of invagination. (a-b) Pattern of specific cell shape changes define tissue-level morphogenesis. (a) Mesoderm invagination in Drosophila melanogaster. Colored cells are undergoing specific cell shape changes (including but not limited to apical constriction). (b) During chick neurulation, bending of the neural plate into a neural tube is achieved through apical constriction in specific regions of the neural plate (red). Pattern of cell shape changes define tissue-level morphogenesis. (c-d) Schematic representation of the effect of apical constriction on epithelial morphogenesis.
Despite a good deal of study, a complete explanation of the basic cytoskeletal, force-generating mechanisms that drive the cellular and global shape changes during invaginations have remained elusive. In a case-study of ascidian neural plate invagination, we tested the long-standing apical constriction hypothesis, and more generally tested whether differential localized tensions on cell boundaries are sufficient to drive the process.
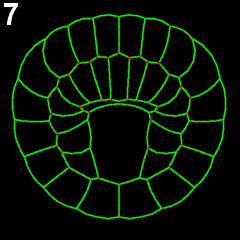
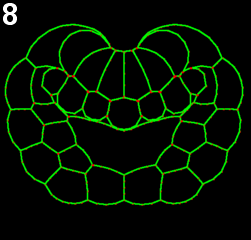
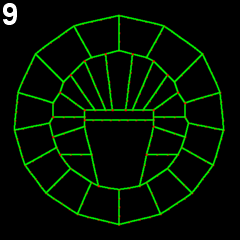
We employed several complementary methods to study neural tube formation, characterizing the process in control embryos, in anterior and posterior explants, and using computational simulations of a two-dimensional cross-section through the neural plate and surrounding tissues. In control embryos, the neural plate forms during gastrulation by a series of directed cleavages that extends the plate in the anterior direction (figure 2, blue shading), taking about 1 hour (all times refer to develop at c. 18 °C). In cross-section, the progressive shrinking of the blastopore is apparent. At the start of neurulation the neural plate begins to invaginate at the posterior near the blastopore edge. The cross sections show how this invagination deepens as the neural plate rolls into a tube (figure 2, 3). Invagination progresses from the posterior of the neural plate towards the anterior, as shown in this fly-through movie of a confocal stack from the embryo shown (figure 4 and movie 2). By 75 minutes into neurulation, most of the neural tube has begun to invaginate, but the tube has not yet begun to close.
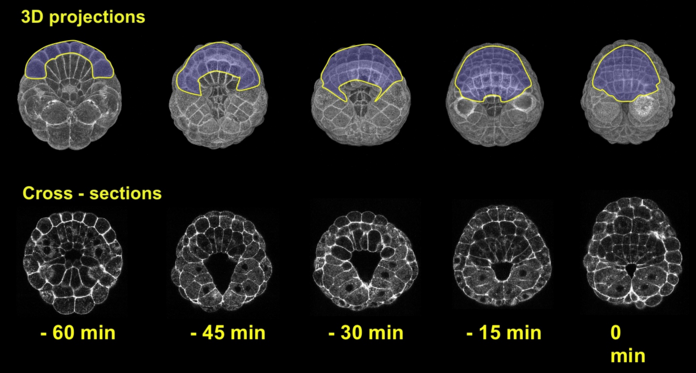
Figure 2: Early morphogenesis of the neural plate. A-line and a-line neural cells (blue) originate from a primordium of 16 cells at the early gastrula stage (left) and make up a 6*8 chessboard structure at the 6-rows stage (also called late gastrula stage). Embryos were fixed from early to late gastrula stage. Chronological sequence goes from left to right. Upper panel represents vegetal 3d projection of confocal stacks of embryos fixed and stained with phallicidin (labels actin); lower panel represents a frontal section of the same embryo.
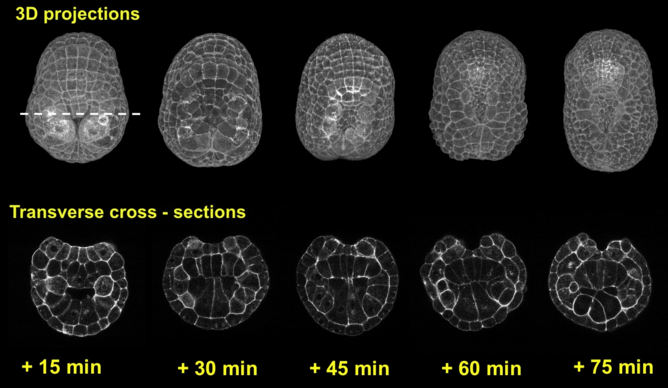
Figure 3: Invagination of A-line neural cells during early neurulation. Embryos were fixed from late gastrula stage to mid neurula stage. Chronological sequence goes from left to right. Upper panel represents vegetal 3d projection of confocal stacks of embryos fixed and stained with phallicidin (labels actin), anterior is up; lower panel represents a frontal section of the same embryos, dorsal is up.
Explanting anterior and posterior halves of embryos during early gastrulation (figure 5) tests to what extent the anterior can autonomously generate the neural plate invagination, and likewise to what extent the posterior can autonomously achieve epithelial closure. Cell fates for the relevant primordia have been determined before gastrulation begins, and are not affected by these experiments. This allows us to study the mechanical effects of isolating parts of the embryo.
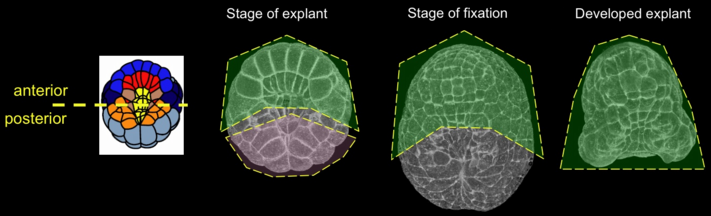
Figure 5: Schematic description of the explant procedure. Left panel, diagram of the axis of cut; other panels represent vegetal 3d-projections of embryos fixed and labeled with phalloidin. Explanted region is outlined (anterior explant, green; posterior explant, colorless). Middle left panel, time of explant; middle right panel, stage of fixation in a control embryo (green, region corresponding to the anterior explant); right panel, anterior explant.
Isolated anterior explants fixed 50 minutes into neurulation were found to have invaginated normally despite lacking mechanical linkage to the posterior (movie 3). Anterior explants labeled with the membrane dye FM4-64 and imaged on the CARV spinning disk confocal were likewise observed to invaginate the neural plate normally, but the invaginated epethelium did not close to form a neural tube (movie 4). Anterior explants mounted in cross-section reveal how timing of neural plate invagination coincides with shape changes in the underlying notochord (movie 5). The neural tube (top) begins to invaginate and shortly thereafter, many cells divide. Subsequently, the neural plate’s invagination deepens and both neural plate and notochord cells columnarize and their adjoining basal surfaces constrict. The basal edges of the neural cells appear to have crawling activity. Finally, the notochord begins its invagination (Munro and Odell 2002) by constricting its apical (lower) surface while its basal surface (facing the neural plate) expands. In control embryos at this stage, the posterior section of the neural tube would already have been sealed; in anterior explants, the still-open neural tube instead bulges outwards, likely due to forces from the notochord. At the end of the movie, the notochord has begun to push outwards as it begins its convergent extension towards the embryo posterior.
What then can the embryo posterior half accomplish in the absence of the anterior? Posterior explants isolated at the early gastrula stage were independently able to complete epithelial closure (“zippering”) to their anterior margins (movie 6). Given the discovery made by others in this course that a contractile V just ahead of the zipper is required for closure to progress (see Shelby Semon abstract), the completion of closure in posterior explants is noteworthy. We conclude that the force of invagination is intrinsic to the embryo anterior while the movements that generate closure initiate in the posterior.
The last element of my study was a computational model of how differential tensions could generate shape changes associated with neural plate invagination (using Morphogenie). The explant results show that neurulation is autonomous in the anterior half of the embryo. Starting with a cross-section representing the embryo before invagination, we searched for localized cortical tensions that generated neural plate invaginations of similar depth to that seen in real embryos. (figure 6). Only high apical neural tensions were able to generate invaginations (movie 7) in agreement with the apical constriction model of invagination. However, in all such invaginations, the notochord was inappropriately short and wide (movie 8). Conversely, if notochord cells were constrained to be normally columnar, neural plate invagination could not occur (movie 9).
Movie 7: High apical tension of neural plate cells is the only path to neural plate invagination. In the modeled geometry, neural plate invagination only occurred in the parameter domain of high apical tension in neural plate cells.
Movie 8: Successful invagination of the neural plate is associated with abnormal notochord morphology. Movie shows runs of the model successfully tested for neural plate invagination. Successful runs all present abnormal geometry with short and wide notochord, incompatible with notochord invagination observed in vivo.
Movie 9: Forcing the notochord to invaginate reverts invagination of the neural plate. After neural plate invagination, if notochord parameters are set to drive notochord invagination, neural plate invagination is reverted, as observed in anterior explants.
We suspect that several additional factors are at work that we have not yet represented in our simulations. First, other cells in the embryo are undergoing morphogenetic movements at the same time as neural plate cells are invaginating. For example, the epidermal cells are cleaving directionally to extend the embryo anterior-posteriorly (see Brooke Danaher abstract) and the notochord cells are themselves invaginating in the opposite direction as the neural plate cells (towards the ventral pole) and converging. Our movies of anterior explants suggest that the timing of these invaginations is offset, with the neural plate invagination occurring first (at least in the embryo posterior) and the notochord invagination somewhat later (movie 5). This difference in timing may be critical for achieving oppositely-directed invaginations in these basally-adjoining tissues. In support of this idea, we simulated neural plate invagination with one set of tension parameters while allowing the notochord to be stretched laterally, then increased cortical tensions on the apical and basal notochord surfaces to make the notochord columnarize. Under these conditions the neural plate invagination begins to push outwards, just as in the live explants. The second factor currently lacking in our simulations is a means of representing active cell crawling. We suspect this may play a major role in the neural plate invagination, both because we have seen signs of basal crawling in the cross-sectional movies, and because of evidence gathered by other students in this course that apical contractility may not be driving neural plate invagination. While the neural plate cells do apically shrink coincident with their invagination (see Diana White’s abstract), we find no actively contractile phospho-myosin on the invaginating neural plate cells (see Monica Montgomery’s and Shelby Semon’s abstracts). We are confident that we would have seen it if it were there, as we have observed phospho-myosin associated with apical constriction of invaginating endoderm cells (See Kristin Sherrard’s page) cleavage furrows, blastopore closure, and formation and progression of the “zipper”. Finally, Montgomery and Semon both found that the Rho-kinase inhibitor Y-27632, which interferes with numerous forms of actomyosin contractility, including invagination of the endoderm during gastrulation (Sherrard, Robin et al. in prep.), does not prevent neural plate invagination (again, see Monica Montgomery’s and Shelby Semon’s abstracts).
We find that neural plate invagination in ascidians represents an intriguing departure from the dogma that differential tensions, and in particular active apical constriction, drive invaginations. Although it does appear to be autonomous to the anterior half of the embryo and to occur independently of zippering, it seems to occur by some other mechanism than active apical contraction and to be carefully coordinated with potentially [clashing] shape changes in the notochord cells. We plan to use fluorescently-labeled gap43 and Rac constructs to look for stronger evidence of basal neural cell crawling, and to modify the simulation in order to allow for this. The invagination of the ascidian neural plate should be a fruitful addition to other well-studied invaginations, and given its simplicity and large cell size should provide unparalleled information about the cellular basis for neural invagination in particular.