(Monica Montgomery)
Ascidian neural tube closure (zippering) occurs by two distinct steps: first, during early neurulation cells and materials assemble at a posterior center we call the zipper origin ( movie 1 ). Subsequently, the neural tube begins to seal uni-directionally towards the anterior of the embryo ( movie 2 ) (also see Shelby Semon’s abstract).
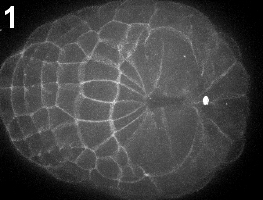
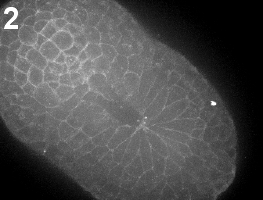
Movie 1-2: Initiation of the zipper. Time-lapse confocal imaging of embryos labeled with the membrane dye FM4-64. The process can be divided into two subsequent steps: initiation of the zipper (Movie 1) and zippering (Movie 2). Vegetal view, anterior is left.
The first step of building the zipper origin may be required for neural tube closure, as suggested by the failure of Joshi’s anterior explants to close . Here, we characterize the cell behaviors associated with building the zipper and explore the role of contractility using immunostaining with mono- and di-phosphorylated myosin regulatory light chain (MRLC) antibody and inhibition of rho-kinase with the drug Y-27632.
We began identifying the cell types in contact with the zipper origin and their associated shape changes. By the early mid-neurula stage ( movie 3 ), many cells have gathered towards the zipper origin, which is in the vicinity of two clots of actin along the posterior midline (figure 1).
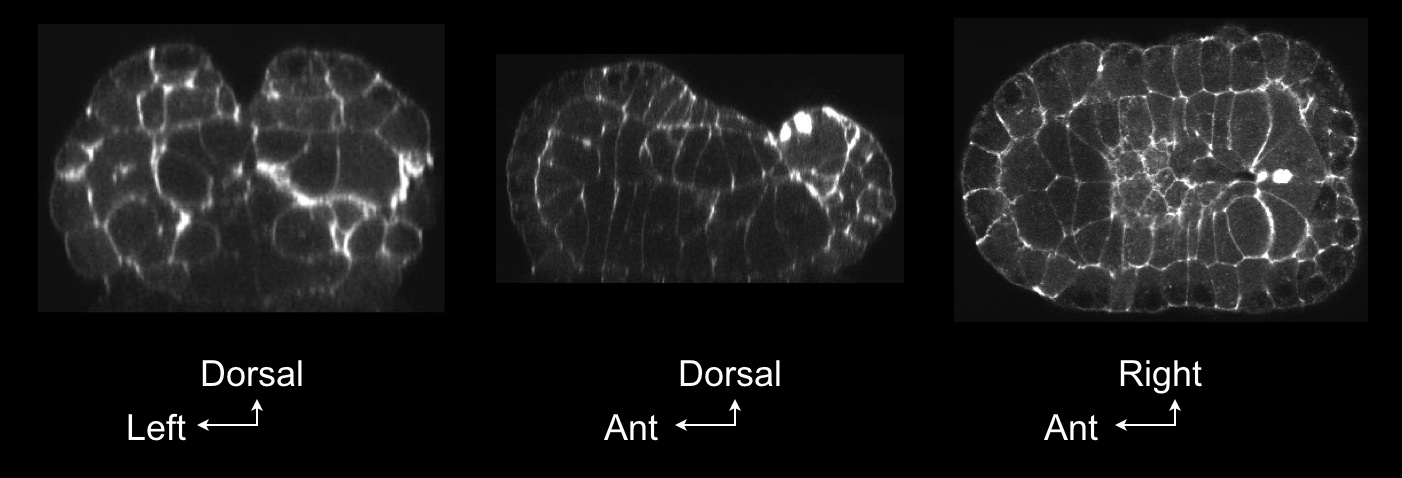
Figure 1: Confocal view of the building of the zipper. Cross-section through Ciona embryo at the mid-neurula stage during the building of the zipper shows accumulation of actin in clots (arrows). Embryos were fixed at the mid-neurula stage and stained with phalloidin. Left, frontal view; middle, sagittal view; right, transverse view.
We reconstructed this embryo using the software Amira and 3-D Virtual Embryo (Tassy et al, 2006), and found that muscle (orange), notochord (red), and epidermal cells (white) are cinched in towards this center, though neural plate cells (blue) are not (figure 2).
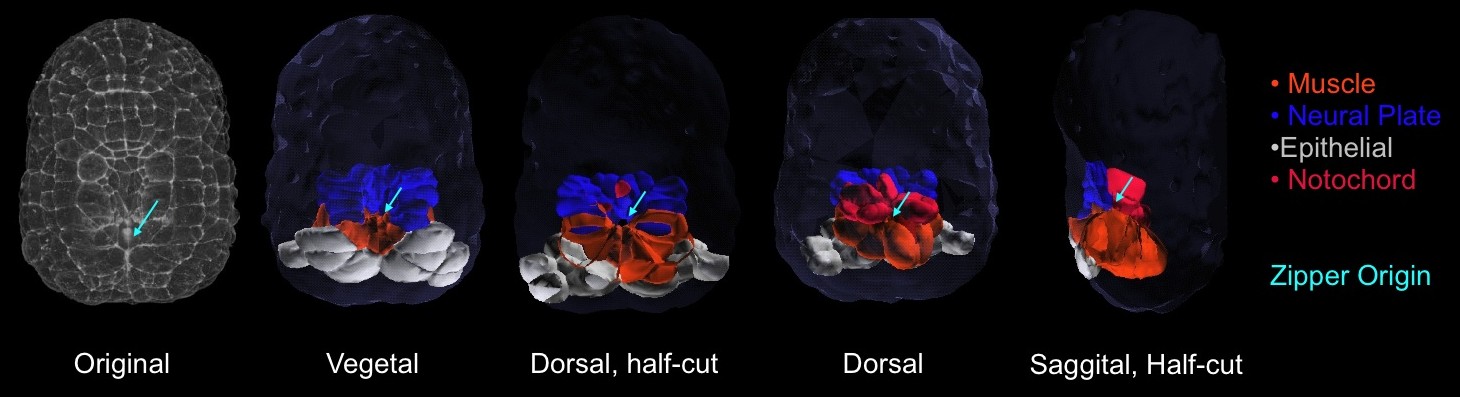
Figure 2: 3-dimensional reconstruction of the embryo during building of the zipper. . From left to right: vegetal projection of confocal stack; vegetal view of 3d-reconstructed embryos and transverse section; animal view and transverse section; sagittal section.
With time-lapse imaging and confocal microscopy of live and fixed embryos, we show that initiation of neural tube closure occurs by three main events: the first, is cinching of muscle cells lining the posterior blastopore towards the closing blastopore. ( figure 3 ). Second is the rolling-inward of the two posterior-most roof cells along the embryo’s midline ( figure 4 , movie 4 ). The rapid reduction of these cells’ apical surfaces is suggestive of a contractile process. The third is crawling of posterior epithelial cells towards the zipper origin ( figure 4 , movie 4 ).
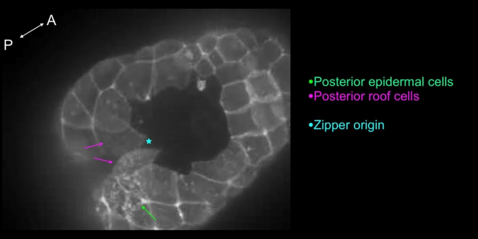
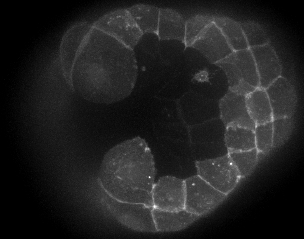
Figure 4 - Movie 4: Crawling of epidermal cells and rolling in of roof cells associated with the initiation of the zipper.
We went on to test the hypothesis that the gathering of cells and materials at the zipper origin are significantly driven by myosin contractility. Using immunostaining, we found both f-actin (stained with phallacidin) and monophosphorylated nonmuscle myosin II (one of two actively contractile forms of non-muscle myosin II) at the blastopore margin during blastopore closure and at the point where cells are cinching in the posterior ( figure 5 ). We also found diphosphorylated nonmuscle myosin II (the other actively contractile form, which is often associated with stable, high-tension structures) concentrated at the zipper origin at earlier and later stages corresponding to the time of initiation of neural tube closure ( figure 6 ). Thus, we see actively contractile myosin at the exact time and location of blastopore closure and building of the zipper origin.
We were able to inhibit rho-kinase-activated myosin contractility using the drug Y-27632, and this had major effects on formation of the zipper origin. Embryos treated with Y-27632 during zipper formation failed to begin zippering ( figure 7 ). Timelapse of Y-27632-treated embryos reveals that inhibition of rho-kinase-mediated contractility causes a drastic disassembly of material already collected at the zipper origin and significant deformation of zipper-initiating cells ( movie 5 ). Thus myosin contractility is essential for the initiation of neural tube closure.
Several aspects of zipper formation are of particular interest and merit further exploration. First, the building of the zipper is closely linked in time and space to blastopore closure, but involves two clots of actomyosin, one at the posterior midline of the blastopore and one further back ( figure 5 ). These two clots merge anteriorwards just before zippering begins. The anterior-most one is on the apices of the CAB cells (the pair of cells containing the centrosome-attracting body, involved in asymmetric cleavages and cell fate determination during early embryogenesis). As this structure may be ablated easily later on without affecting early embryogenesis, we may test whether it is required for neural tube closure. Finally, the combination of cell crawling and extremely localized contractility is intriguing—does the contractility direct the crawling towards the zipper origin? Do the tiny clots of myosin continually reel in actin filaments to drive the extensive shrinkage of apical surfaces? Future experiments include laser-ablating the CAB cells and, separately, the posterior-midline muscle cells, to observe the effect on zipper formation, immunostaining of more controls and ofY-27632 (rho-kinase inhibited) embryos with phospho-myosin to characterize the extent of loss of contractility, and sequential 3D reconstructions of zipper origin-associated cells from late gastrula to mid-neurula to track cell movement and cell shape change in the building of the zipper.