(Shelby Semon)
Following invagination of the neural plate, in Ascidians, the lips of the invaginated neural primordium close to form a tube. Neural tube closure occurs in two distinct steps: first, during early neurulation cells move towards a posterior center we call the zipper origin (movie 1). Subsequently, the neural tube begins to seal closed, in a unidirectional process we call “zippering”, which propagates from the embryo posterior towards the anterior (movie 2).
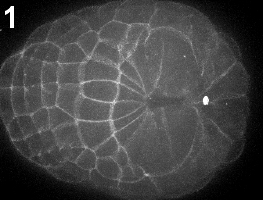
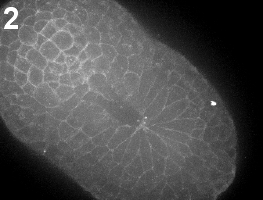
Movie 1-2: Time-lapse movies of early and late neurulation. Vegetal view of Ciona intestinalis embryo stained with FM4-64. Anterior is left. Movie 1, building of the zipper; movie 2, closure of the neural tube by antero-posterior zippering.
Zippering entails three essential cell behaviors: cell crawling (movie 3), cell-cell adhesion (movie 4) and cortical contraction (movie 5). Using time-lapse microcopy we discovered that zippering involves two layers of closing cells (movie 6), one neural, one epidermal. In investigating the possible role of actomyosin contractility in zippering, we discovered a mechanically continuous actin purse string surrounding the neural plate (figure 1, movie 7).
To quantify the kinematics of zippering, we measured the region of cell-cell contact at the neural/epidermal boundary. We found that cell boundary shrinkage was maximal at the zipper and propagated up to two cells ahead of the zipper, implying that contraction is maximal at the zipper and decreases further away from it (figure 2-3).
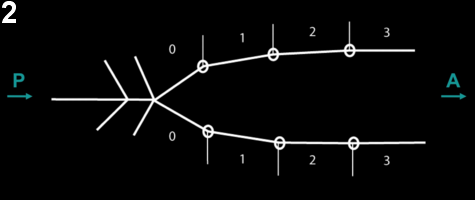
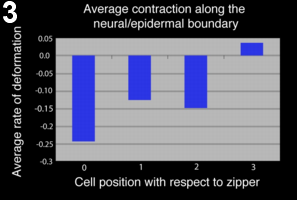
Figure 2: Schematic of the measure of contraction of the neuro-epidermal boundary ahead of the zipper. On the left side, neural folds have fused. On the right, cells are numbered starting from the zipper. The length of the segment represents the length of the apical junction between neural and epidermal cells.
Figure 3: Neuro-epidermal boundary contracts up to 2 cells ahead of the zipper.
Using immuno-staining, we sought to we determine where and when myosin was activated during neural tube closure. We found that ser19-phosphorylated myosin, an activated form of myosin II, localized to the boundary cells closest to the zipper, forming a myosin-enriched structure we call the “V”. Thus, the active myosin pattern corresponds to the area of active shrinkage observed in the kinematics study (figure 4).
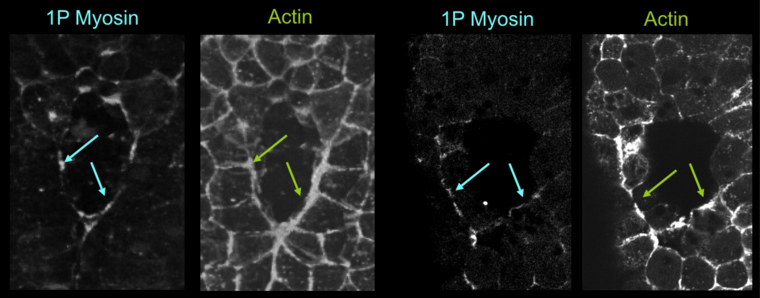
Figure 4: Monophospho-myosin pattern during zippering. Embryos were fixed at the mid-neurula stage, subsequently stained for actin (phallicidin, right and middle left panel) and Monophospho-myosin (left and middle right panel), and imaged by confocal microscopy. Monophospho-myosin accumulates specifically along the neuro-epidermal apical junctions close to the zipper. Frontal view, anterior is up.
To better understand the role of cortical contractility, we used the chemical inhibitor Y-27632 - a drug that specifically inhibits Rho Kinase-activated myosin - to test its effect on contractility at different stages of zipper closure. Embryos treated after zippering had started and fixed one hour later showed an overall normal morphology, except that the neural tube, though invaginated, did not close (figure 5).
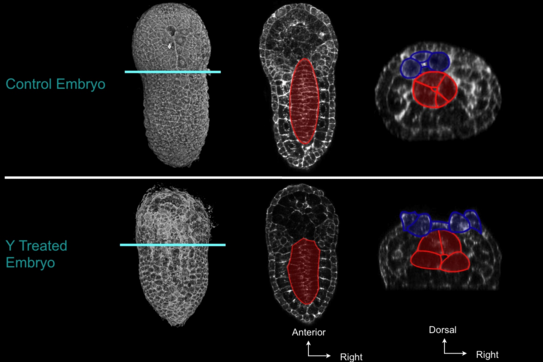
Figure 5: Effect of RhoKinase inhibition on zipper. Embryos treated at the early neurula stage were fixed at the late-neurula stage. The general embryonic morphology is largely preserved, but neural folds did not fuse. Red, notochord; dark blue, neural cells. Left, 3d projection, vegetal view, anterior to the top; middle: frontal view, anterior to the top; right, frontal view, dorsal to the top. On the right panel, note that neural tube is not closed.
To better understand the effect of this drug on neural tube closure, we made time-lapse confocal microscopy movies of Y-27632-treated embryos. We found that when Y-27632 was added near the beginning of the zippering process, zippering did not proceed (movie 7-8). When added late in the zippering process, the zipper broke and the closed region of the neural tube re-opened (movie 9). Since invagination of the floor cells of the neural plate occurred normally in Y-27632 treated embryos, we conclude that Rho Kinase-activated contractility is essential for zipper initiation and propagation, but not for the neural plate invagination that precedes zippering.