
CCD Research Intern
(working with Kerry Kim, Ph.D.)
After graduating with a B.Sc. in Biology and a Mathematics Minor, I came,
in September 2006, to the CCD to participate in the Computational Modeling
of Complex Networks Apprenticeship. I had never worked in biology at this
level. The first diagram I saw of a real genetic network (e.g. the segment
polarity or neurogenic network) raised many more questions than it
answered. How do these tangled hairballs of cross-regulatory interactions,
with no obvious start or end, work? How could these cobwebs of nonsense
stabilize complex spatial patterns of gene expression so essential to
development that without them fruit fly larval bodies, or human ones,
would not exist? Other networks with equally cryptic topologies set up
oscillations that drive cell cycles (Endocycle and cell cycle networks)
and in turn growth. Equally worrying to me - How is it possible for any
human to make sense of this complexity?
Working with Dr. Kerry Kim over the past year taught me how to think about these questions, and more importantly, has raised other even more interesting questions.
An amazing characteristic of regulatory gene networks is their ability to maintain functions in the face of genetic variation and environmental perturbations. Sexual diploid organisms shuffle and throw together components of networks that are unlikely ever to have combined with each other before. Yet, in most cases, these components are able to work together to achieve their complex tasks. I am interested in the general characteristics of networks that may confer robustness. I am especially interested in understanding whether and how diploid versions differ from haploid versions in regards to robustness to environmental and/or genetic perturbations.
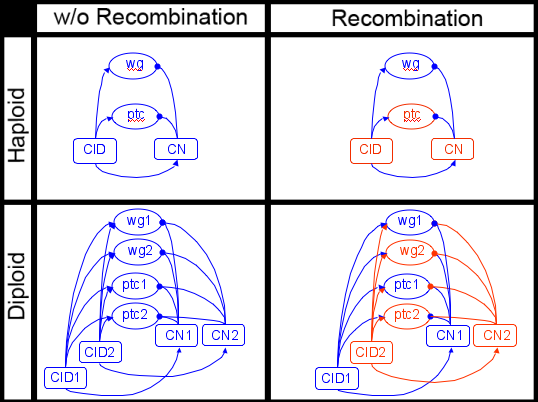
A small section of the segment polarity network generalized from Haploid to Diploid, with and without recombination. Different colors represent components inherited from different parents. Notice how in the diploid case the number of possible interactions quadruples rather than doubles in some instances. <
Diploidy is more than just a mere doubling of gene copies, in many cases it is a quadrupling in the number of interactions between network components. To address this, Dr. Kim and I have generalized von Dassow's haploid Drosophila Segment Polarity Network model that is parameterized to describe the interactions between network nodes. Our extension uses a mutable surrogate for DNA sequence to realistically and mechanistically determine the quantitative traits of each gene product. This framework enables us to map genetic variation at individual loci to kinetic parameters and rate constants that describe the interactions of network components and in turn phenotype. This genotype-phenotype map enables us to generalise the haploid model to a diploid version and correctly enforce recombination with heritable parameters.
Many famous networks occur in widely diverged species. I am interested in examining how evolution might alter gene networks. Are networks such as the Segment Polarity Network, conserved among different species because of the power of its robustness or because evolution has a hard time changing such networks without breaking them? A biological species is a group of organisms capable of interbreeding and producing fertile offspring. In large measure, the incompatibilities delineating species arise from failures of the components of one species to work and communicate with those of another species. Genetic disorders including diseases caused by malfunctioning networks and speciation events caused by incompatible networks are distinct phenomena but have much in common. They are both ultimately the result of broken networks. I wonder if there are insights into genetic disorders and disease to be gained from studying the incompatibilities of networks between closely related species and similarly if insights into speciation may fall out of the study of breakdowns within a species.
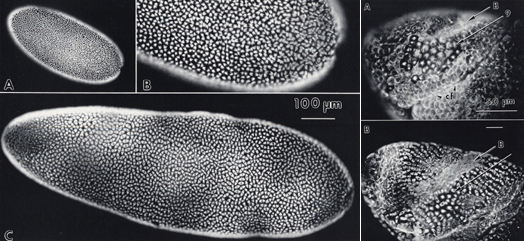
Micrographs of Drosophila (upper panels) and Calliphora (lower panels) stained with fluorescently labeled antibody for β-tubulin show all the nuclei synchronously in metaphase of the 13th division cycle. Panel A (upper left) of an entire Drosophila egg and panel C (bottom left) of an entire Calliphora egg at the same magnification. The right most panels show the ventral-lateral views of the region in front of the cephalic fold in Drosophila (upper) and Calliphora (lower). Caliphora is imaged at three times lower magnification but shows the same mitotic domains as Drosophila. Photo Credit: Victoria Foe and Garrett Odell. 1989. Mitotic Domains Partition Fly Embryos, Reflecting Early Cell Biological Consequences of Determination in Progress. American Zoologist: 29(2):617-652
I am currently exploring how variations in the size of cells influence network behavior. For example, blowfly embryos have exactly the same number of cells as Drosophila embryos, and develop on the same time schedule up to hatching. But each cycle-14 blowfly cell has several times the volume of its Drosophila counterpart. How, with only the same two copies of each gene, do the blowfly cells fill their vastly greater volume with an adequate concentration of each protein?