Soichiro Yamada and W. James Nelson
The long term goals of our work are to understand how epithelial cells organize into monolayers through specialized cadherin-mediated cell-cell contacts, and localize proteins to functionally different plasma membrane domains. We integrate different experimental approaches to address these problems: structural analysis of proteins and protein complexes, high resolution live cell imaging, biochemical analysis of protein complex assembly and function in cells, and in vitro reconstitution of protein functions. We have defined stages in cell-cell adhesion and obtained preliminary evidence of a mechanism involved, determined how several plasma membrane proteins are targeted to and organized in specific membrane domains, and developed new in vitro methods to dissect protein interactions and functions at cell-cell contact plasma membranes. controlling membrane domain formation by cadherin-based cell-cell adhesions. These results begin to define molecular linkages and mechanisms controlling membrane domain formation by cadherin-based cell-cell adhesions.
We discuss 3 experimental approaches, each providing a different angle of insight into protein localization, interactions and, potentially, function. Our aim is to combine the overlapping information derived from each approach to generate a more complete picture, and to use information obtained from one approach to design new experiments using another approach.
We use high resolution, time-lapse imaging and FRAP analysis of GFP fusion proteins (e.g., E-cadherin, actin, Rac1, PH-AKT, etc) to describe stages in cell-cell adhesion, and then mutant proteins and pharmacological interventions to investigate underlying mechanisms. We find that as migratory MDCK cells collide, lamellipodia increase in number, perdurance and concentration at cell-cell contacts, coincident with localization of Rac1 and PH-AKT (marker for PI(3,4,5)P3) at edges of the expanding contact, indicating that cadherin engagement results in local activation of Rac1, perhaps through recruitment of GEFs. Immediately, a highly mobile, diffuse pool of E-cadherin coalesces between contacting membranes into immobile puncta that are spatially coincident with membrane attachment sites for actin filaments (Fig. 1). Subsequently, circumferential actin cables underneath the forming contact separate (Fig. 1), and the resulting ends of the cable together with a subset of E-cadherin puncta are swept laterally resulting in gradual maximization of the contact length. Based on studies with inhibitors of Rho-kinase (Y27632) and MLCK (ML-7), RhoA activation of actin-myosin contraction at the edges of thee contact may be important for this later stage of cell-cell adhesion that lead to compaction.
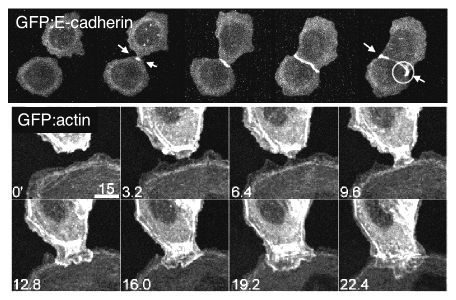
Figure 1: Time-lapse fluorescence microscopy of MDCK cells expressing E-cadherin/GFP (top) or actin/GFP (bottom) at different times during cell-cell adhesion.
E-cadherin-mediated cell-cell adhesion leads to the rapid formation of a new membrane domain at the site of contact, and the eventual development of cellular polarity. Formation of a new membrane domain, apical or basal-lateral requires targeting of transport vesicles from the Golgi to that membrane domain. We focused on the link between cell-cell adhesion and formation of a targeting patch to specify basal-lateral vesicle delivery to that site. The exocyst/Sec6/8 complex is a multi-protein complex essential for targeting exocytic vesicles to specific docking sites on the plasma membrane of polarized epithelial cells. E-cadherin-mediated cell-cell adhesion results in rapid recruitment (t1/2 3-6 h) of ~70% of the Sec6/8 complex to sites of cell-cell contact. To examine protein complexes involved in Sec6/8 recruitment, we have been able to fractionate membrane microdomains in OptiPrep density gradients, resulting in the separation of apical membranes, tight junctions, and the remainder of the lateral membrane (Fig. 2 shows distributions of Sec6, E-cadherin and nectin at early (6hrs) and late (48hrs) times after cell-cell adhesion; note change in Sec8 distribution to membranes containing nectin and E-cadherin). Protein complexes can be further resolved by solubilizing membranes in detergent-containing buffer, and proteins identified directly by immunoprecipitation and western blotting, or following a subsequent separation step by Superose 6 FPLC. The combination of these approaches has lead to the identification of E-cadherin and nectin 2a as membrane binding sites for the Sec6/8 complex. This consistent with the requirement for cadherin-mediated adhesion in both recruited of the complex to sites of cell-cell adhesion, and studies for other laboratories showing that cadherin-mediated adhesion leads to delivery of basal-lateral transport vesicles to those membrane sites.
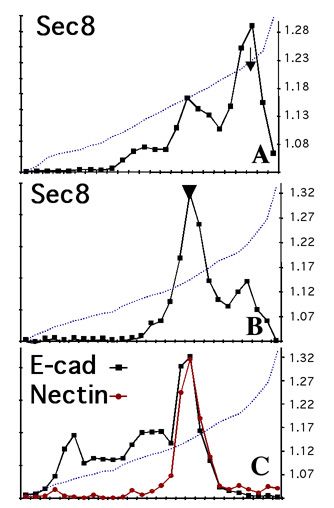
Figure 2: MDCK membranes separated in OptiPrep gradients (self-forming from a 10-20-30% step gradient) 6hrs (A) and 48hrs (B, C) after cell-cell adhesion; after SDS-PAGE, western blotting showed recruitment of Sec8 from a large cytosolic pool (arrow) to membranes containing adhesion proteins E-cadherin and nectin (arrowhead).
While there has been progress in identifying protein complexes and correlating activities with events at cell-cell contacts, we are still limited in our ability to biochemically define protein assembly pathways and functions. We have developed and characterized a new method that will enable us to reconstitute and molecularly dissect cytoskeleton (catenins, actin filaments) and targeting patch (Sec6/8 complex, SNARE proteins) assembly and function at E-cadherin-mediated adhesion sites. The basis for this method is the attachment of MDCK cells to a correctly oriented dense E-cadherin substrate and the in situ isolation of open lateral plasma membrane patches attached to the E-cadherin substratum. Dense, correctly oriented and functional E-cadherin substratum is prepared as described in Fig.3. MDCK cells rapidly attach, spread using lamellipodia, and flatten on the FcE-cadherin substratum within ~5hrs (Fig. 4, top). Immunofluorescence shows that cell-FcE-cadherin bound membrane contains proteins characteristic of lateral membranes (E-cadherin, catenins, ZO-1, Na/K-ATPase, fodrin, Sec6/8 complex), but not of basal (paxillin, integrins) or apical (gp135) membranes.
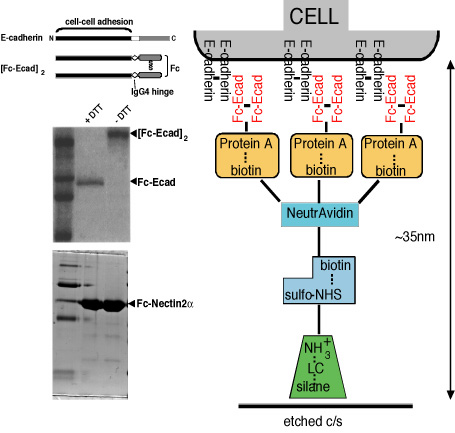
Figure 3: Preparation of FcE-cadherin substrate.
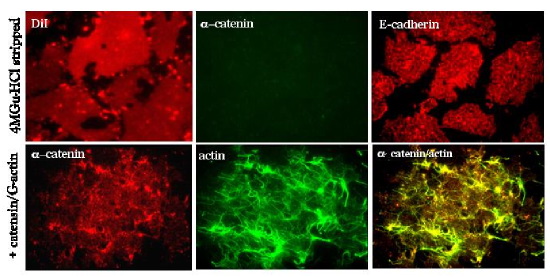
Figure 4: Immunofluorescence of MDCK lateral membrane patches attached to EcadFc substratum. Top, Patches were stripped with 4M guanidine-HCl, note patches are intact (DiI), stripped of a-catenin (center), but transmembrane E-cadherin (right) remains; Bottom, Stripped patches were incubated with cytosol as a source of catenins which bind (left) to E-cadherin (not shown), and then Fl-labeled G-actin (center) was added at RToC for 15', note actin filaments have polymerized and appear to be concentrated at membrane spots of reconstituted (a-) catenins/E-cadherin complexes (right).
To prepare open lateral plasma membrane patches, cells are treated to a short sonication pulse which shears off apical membranes, nuclei and intracellular organelles, leaving behind open lateral plasma membrane patches attached to the FcE-cadherin substratum. Lateral plasma membrane patches labeled with the lipid dye DiI show a characteristic, homogeneous patch-like pattern (Figure 4), and staining with E-cadherin and catenins and other proteins characteristic of the lateral membrane (not shown) domain reveal punctate and diffuse staining over the patches. Membrane-associated proteins such as actin/a- and b-catenin are stripped from membrane patches with 4M guanidine-HCl, but membrane patches and E-cadherin remain intact (Fig. 4). Incubation with cytosol leads to the re-addition of a- and b-catenin to E-cadherin sites (Fig. 4); note that E-cadherin cytoplasmic domain is naturally unstructured, and adopts an ordered secondary structure only after binding to b-catenin. Incubation of lateral membrane patches with fluorescein-labeled G-actin leads to formation of long actin filaments that appear to originate from E-cadherin/catenin clusters (Fig. 4). Protein complexes have now been assembled from purified proteins.
We have adapted these techniques further: 1). Basal membrane patches attached to extracellular matrix to study microtubule-plasma membrane interactions and organization; 2). Imaging and biochemical manipulation of exocytic transport vesicle docking and fusing with cadherin-based membrane patches (role of Sec6/8 complex and syntaxin); 3). Formation of micro-patterned chimeric substrata containing extracellular matrix surrounding fixed or lipid bilayer supports containing cadherin.
cytomechanical modules 2003 • back