Vladimir Rodionov
Polarized radial arrays of cytoplasmic microtubules (MTs) with minus ends clustered at the cell center define the organization of the cytoplasm through interaction with molecular motors bound to the membrane organelles or chromosomes. MTs in the array serve as 'rails' for motor-mediated transport between membrane compartments and as a structural basis for organelle positioning in the cytoplasm. Formation of the radial MT array is generally assumed to be a result of nucleation at the centrosome, which often occupies position at the center of a cell. Recent studies indicate, however, that cells have a remarkable self-organizing capacity, that they can organize a radial array of MTs in the absence of the centrosome and that mechanisms exist that position a focal point of converging MTs in the center of a cell. Here we examine the mechanisms of self-organization and self-centering of a radial MT array.
To understand how MTs self-organize into a radial array we use cytoplasmic fragments of melanophores as an experimental system. In melanophores, thousands of pigment granules rapidly aggregate to the center or redisperse uniformly throughout the cytoplasm. The granules move by means of the minus-end-directed MT motor cytoplasmic dynein (aggregation) or a plus-end-directed motor of the kinesin family (dispersion). Microsurgically produced cytoplasmic fragments of melanophores lacking the centrosome rapidly form a polarized radial array after the stimulation of minus-end directed movement with adrenaline and position the pigment aggregate at the focal point of the converging MTs (Fig. 1).
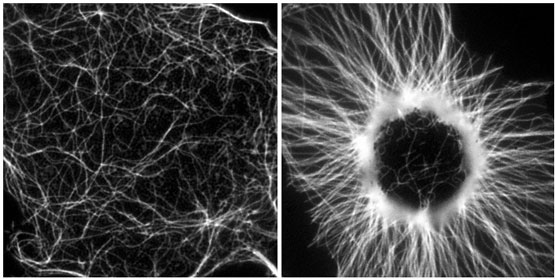
Figure 1. Live fluoresecence images of MTs in melanophore fragment before (left) and after (right) stimulation with adrenalin of dynein motors bound to pigment granules. Random MTs organize into a regular radial array.
We demonstrate that organization of MTs into a polarized radial array involves their continuous disassembly and reassembly. Growth of MTs is initiated on pigment granules that provide nucleation templates, but unlike the centrosome do not anchor the minus ends tightly. Frequent release and depolymerization from minus ends allows for rapid reorganization of a MT array. The spatial distribution of MTs at any given moment of time is determined by the location of pigment granules. However, pigment granules themselves do not remain stationary but are rapidly transported to the MT minus ends. Nucleation and frequent release of MTs superimposed on minus-end directed transport of the granule-associated nucleation sites eventually result in the formation of a radial array. We find that nucleation of MTs on pigment granules in the fragments is inhibited by dynein inhibitors. Further, we show that purified cytoplasmic dynein nucleates MTs in vitro. We conclude that self-organization mechanism involves MT dynamics and the activity of cytoplasmic dynein, which plays a dual role by participating in MT nucleation and by supporting minus-end directed transport of nucleation sites.
It is known that centrosome positioning requires a radial array of cytoplasmic MTs that can exert pushing or pulling forces involving MT dynamics and the activity of cortical MT motors. It has also been suggested that actomyosin can play a direct or indirect role in this process.
To examine the centering mechanism, we disrupted MTs in BS-C-1 cells by local application of nocodazole, an inhibitor of MT assembly. Such local drug application induced disruption of MTs on the cell edge proximal, but not distal to the application site, thus introducing an imbalance in the MT-related forces applied to the centrosome. Local MT disruption induced rapid movement of the centrosome towards the micropipette in all examined cells. Remarkably, the direction of the movement reversed if cells were pre-treated with inhibitors of actin and myosin contractility, - Rho inhibitor C3-transferase or MLCK inhibitor ML7, indicating that the centrosome positioning is controlled not only by MT, but by actin-dependent forces as well (Fig. 2). The change of the direction of the centrosome displacement in the presence of actomyosin inhibitors further suggests that the MT-dependent force involved in the centering is of pulling rather than pushing nature and that it acts in opposition to the actin-dependent forces. Such MT pulling could be produced by cortical cytoplasmic dynein. Indeed, in cells injected with dynein blocking antibody the centrosome failed to maintain its central position and was misplaced toward the nearest cell margin. Therefore a MT-dependent dynein pulling force plays a key role in the positioning of the centrosome at the cell center, and other forces applied to the centrosomal MTs, including actomyosin contractility, can contribute to this process.
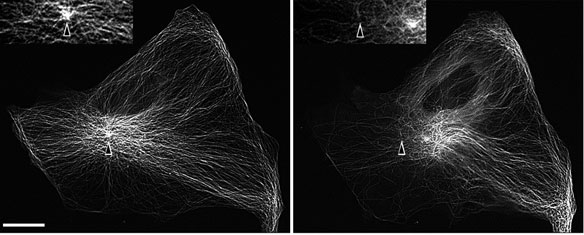
Fig. 2. Fluorescence images of the centrosome in the cell with inhibited RhoA activity before (left ) and after (right) local application of nocodazole. Nocodazole was applied to the left margin. The initial position of the centrosome is indicated by black arrows. The centrosome moves away from the site of nocodazole application.
In conclusion, our results indicate that the MT dynamics and the activity of minus-end directed MT motors are essential for the self-organization and self-centering of a radial MT array. This cytomechanical module requires a surprisingly small number of functional components because cytoplasmic dynein plays multiple important roles in organization and positioning of the MT asters.
cytomechanical modules 2003 • back