Julie Theriot
Polymerizing networks of actin filaments are capable of exerting significant mechanical forces used by eukaryotic cells and their prokaryotic pathogens to change shape or to move. In several biological systems where the energy derived from nonequilibrium actin polymerization is used to generate propulsive force, actin networks demonstrate the ability to perform large-scale self-organization to create a polarized array. We are dissecting the biochemical and biophysical basis of actin network self-organization in several model systems.
The simplest case where a dendritically branched actin filament network can be seen to generate spontaneous large-scale polarity is in the case of spherical polystyrene beads coated uniformly with a protein that catalyzes actin nucleation via the Arp2/3 complex, such as ActA from the intracellular bacterial pathogen Listeria monocytogenes (Fig. 1). Initially the spheres are surrounded by a uniform cloud of actin that can convert to a polarized, motile "comet tail" in a manner that is sensitive to properties of the particle surface including size and surface density of ActA (1).
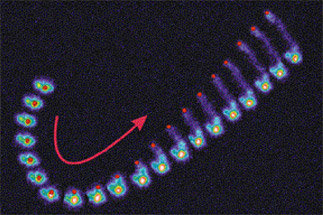
Fig. 1. Composite time series showing spontaneous symmetry-breaking of an actin cloud surrounding a spherical polystyrene bead coated uniformly with ActA. Bead position is shown in red. Actin filament density is shown in pseudocolor with cool colors representing low densities. Frames are separated by 10 sec.
ActA coating can confer motility on artificial particles other than polystyrene beads, including phospholipid vesicles (2, 3). Formation of actin comet tails on vesicle surfaces causes deformation of the vesicle shape. These deformations enable us to estimate the spatial distribution and magnitude of actin-dependent forces on the vesicle surface (Fig. 2). The inward compression force exerted by actin polymerization orthogonal to the direction of motion is >10-fold greater in magnitude than the component of the force exerted in the direction of motion. Furthermore, there is a spatial segregation of the pushing and retarding forces, such that pushing predominates along the sides of the vesicle, although retarding forces predominate at the rear. The dominance of compression forces and the functional separation between pushing and retarding forces are consistent with aspects of both mesoscopic and microscopic biophysical models for force generation in this system (4, 5).

Fig. 2. Deformations of ActA-coated phospholipid vesicles due to actin comet tail formation. A. In the absence of ActA, vesicles assume spherical or prolate shapes. B. After comet tail formation, vesicles are distorted with the comet tail associated with the narrower end. C. Distribution of actin-dependent forces calculated on the vesicle surface (red net inward, blue net outward). From Ref. 2.
Actin-based motility of live L. monocytogenes is persistent and unidirectional, with the rod-shaped bacteria moving parallel to their long axis. In contrast to the simplified artificial particle systems, the bacteria have inherent structural polarity and express the ActA protein at a higher density on one pole. In order to determine whether the characteristic orientation of the bacteria is due to bacterial cell shape or polar expression of ActA, we have compared the motile behavior of spherical polystyrene beads uniformly coated with ActA to the behavior of polystyrene ellipsoidal beads. Ellipsoidal beads of two different aspect ratios were able to form comet tails and move in any orientation. Most beads formed tails either parallel to their long axis or perpendicular to their long axis, with a minority moving diagonally or switching orientations while moving. The average speed for all particles was identical regardless of shape or orientation. This indicates that the polarity of bacterial motility must be based primarily on the underlying polarity of the ActA protein and not on a physical preference for movement parallel to the long axis.
In the vesicle system describe above, the surface of the particle is fluid as well as deformable, and ActA can redistribute such that it is most highly concentrated at the rear of the vesicle, at the same location where retarding forces dominate (2, 3). In contrast, ActA is thought to be immobile on the surface of L. monocytogenes or polystyrene beads. To examine the contribution of surface fluidity to the establishment of movement polarity, we coated the surface of ellipsoidal beads with a fluid phospholipid bilayer prior to addition of ActA. Surprisingly, this treatment resulted in a new emergent phenotype not previously seen with any artificial particles: peristent circular movement (Fig. 3). We are currently investigating how the force balances at the particle surface may give rise to this unique steady state.
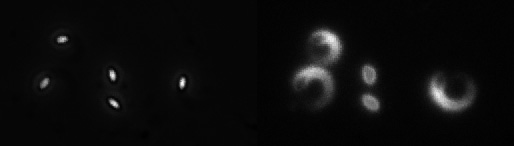
Fig. 3. Persistent circular movement by ellipsoidal beads coated with a phospholipid bilayer. Left, phase contrast image. Right, rhodamine-actin fluorescence. The two particles in the middle have not yet broken symmetry.
A similar dendritic actin meshwork is responsible for the extension of the lamellipodium at the leading edge of motile cells including fish epidermal keratocytes. At steady state, keratocyte movement is persistent and unidirectional and the cells maintain a simple, stereotyped, polar geometry. Maintenance of the motile steady state can be modeled based on assumptions of the balance of protrusion and contraction rates (6) or even with molecular detail (7). As with movement driven by comet tails, the constant polar moving state of keratocytes is preceded by a nonpolar, stationary state. We have developed a technique to isolate large numbers of stationary keratocytes. Stationary keratocytes have a circular morphology. Motile keratocytes have a half-moon shape with a large lamellipodia that carries the cell body on its dorsal surface and are capable of very rapid movement (up to 1 µm/s). Over time, circular stationary keratocytes were observed to break symmetry spontaneously and generate a well-defined and persistent structural polarity in the absence of extrinsic spatial cues. We wished to determine whether spontaneous polarity was generated by specification of the leading edge (as in chemotaxis) or by specification of the trailing edge. Time-lapse phase contrast microscopy indicated that during spontaneous motility initiation the rear of the cell retracted first, followed by forward translocation of the cell body and finally the front cell margin, over a period of ~200-400 seconds (Fig. 4). This suggests that spontaneous motility initiation in keratocytes involves changes in the rear of the cell that are propagated to the leading edge, in contrast to the polarization of chemotactic cells in response to stimulation, where the leading edge is specified first.

Fig. 4. Spontaneous symmetry-breaking and movement initiation in a fish keratocyte. Right, circular stationary cell. Middle, polarity initiation with retraction at the right. Left, fully polarized cell initiating movement. Frames are separated by about 2 min.
cytomechanical modules 2003 • back