Holly Goodson
The process of cell organization underlies fundamental biological processes ranging from polarized growth to multicellular development. Because many aspects of cell organization depend on the microtubule cytoskeleton, a major part of this initally diffuse problem can be reduced to two more tracktable questions: a) How is the microtubule cytoskeleton itself morphologically defined? b) How do other cellular components interact with microtubules? To answer these questions requires knowledge of the proteins that control microtubule (MT) dynamics and mediate cargo-MT interactions. Work in our laboratory is focused on a protein involved in the answers to both of these questions, CLIP-170.
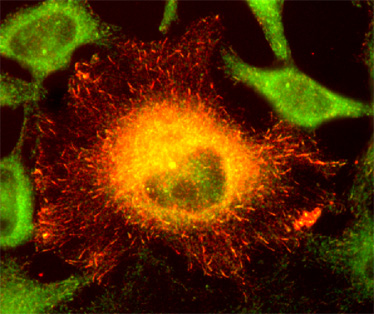
Figure 1. Localization of transfected CLIP-170 in red; endogenous p150 (a related plus-end tracking protein) is in green. (MeOH-fixed HeLa cells)
CLIP-170 is the founding member of a class of proteins with the surprising ability to dynamically track growing MT plus ends (Figure 1). Because of this unusual localization and the understanding that the tubulin conformation at the MT plus-end defines the propensity of the microtubule to grow or shrink (Figure 2), these "microtubule-plus-end tracking proteins" are predicted to regulate microtubule dynamics. Increasing evidence from a variety of sources indicates that they are also involved in mediation of interactions between "the rest of the cell" (membranes, chromosomes, the cell cortex) and microtubules.
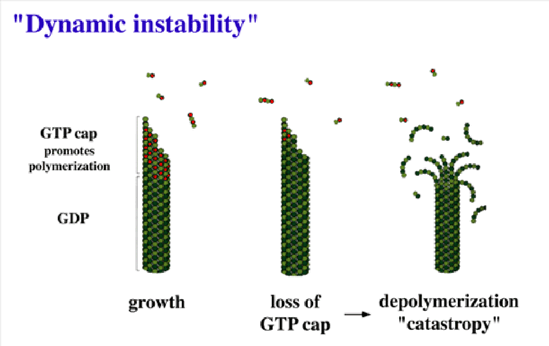
Figure 2. Mechanism of dynamic instability
To begin to address the role of CLIP-170 in these processes, it is necessary to better define its interactions with microtubules. In particular, we are investigating the mechanism of its plus-end tracking activity and its effect of microtubule dynamics. Our experiments indicate that CLIP-170 tracks MT plus ends by first associating with unpolymerized tubulin, then copolymerizing, then falling off of older areas of the polymer, most likely by a mechanism involving CLIP-170 phosphorylation (Figure 3). Our experiments show that CLIP-170 has very high affinity (~30nM) for unpolymerized tubulin and indicate that CLIP-170 is more of a tubulin binding protein than a microtubule binding protein. Though models where CLIP-170 would track MT plus ends by recognizing some plus-end specific conformation (the GTP cap) are attractive, the sum of our data argue against these models. We propose that the preassociation/ copolymerization model is a plausible mechanism by which the large number of unrelated plus-end tracking proteins could accomplish the same remarkable behavior.
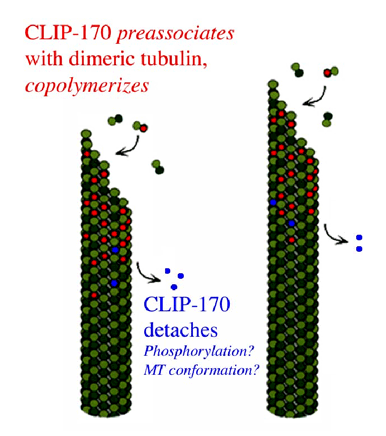
Figure 3. Working model of CLIP-170 plus-end tracking behavior. Note that speckle analysis demonstrates that CLIP-170 does not move on the microtubule.
In vitro dynamic instability assays show that CLIP-170 does indeed alter dynamics of pure tubulin in vitro, suppressing catastrophe and enhancing rescue. These observations are consistent with reported effects of interfering with CLIP-170 function in vivo, and are the first demonstration of a direct effect of a plus end tracking protein on microtubule dynamics.
The idea that membranes can be organized by microtubules is well-established. The observation that CLIP-170 alters microtubule dynamics and is also involved in membrane-microtubule interactions leads to the idea that it may be involved in a system of "organizational feedback", allowing membranous cargo or other subcellular constituents to influence microtubule morphology. Consistent with this idea, plus-end tracking proteins appear to be involved in communication between microtubules and the cell cortex resulting in microtubule capture/selective stabilization. As originally postulated by Mitchison and Kirschner, selective stabilization could be a powerful force for cell polarization and morphological change (Figure 4). There is also evidence that CLIP-170 is involved in global regulation of microtubule dynamics. In summary, microtubule plus-end tracking proteins are physically and biochemically positioned to be a key part of these fundamental organizational processes.
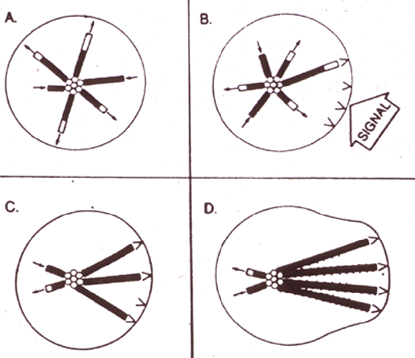
Figure 4. Selective stabilization and morphological change; from Cell 45:329 1986
cytomechanical modules 2003 • back