Geraldine Seydoux
We are interested in the mechanisms that generate asymmetries within cells and are using the C. elegans zygote as a model system. C. elegans zygotes are relatively large, optically clear eggs, which can complete embryogenesis on a microscope slide. Unlike other commonly studied eggs, which develop molecular asymmetries during oogenesis, C. elegans eggs first become polarized after fertilization using cues that arise in the egg itself. In particular, polarity along the anterior-posterior (A/P) axis is determined by the sperm asters, whose position specifies the posterior end of the embryo. In response to this cue, cortical contractions cease in the posterior and several cortically-enriched PAR proteins relocalize to non-overlapping anterior and posterior domains. The PAR proteins in turn regulate the asymmetric distribution of cell fate determinants in the cytoplasm. Polarization takes <10 minutes and is easily followed by time-lapse microcopy.
We focus on several questions, in particular:
Using GFP fusions to follow polarization dynamics in live embryos, we found that polarization involves two genetically and temporally distinct phases (ref 1). During the first phase (establishment), local interactions between the sperm asters and the actin rich cortex exclude the "anterior" PAR proteins (blue in Fig. 1), allowing the "posterior" PAR protein PAR-2 (pink) to accumulate in an expanding domain on the cortex nearest the asters. The second phase begins when the asters assemble the mitotic spindle and begin to invade the anterior. During this phase (maintenance), PAR-2 maintains anterior-posterior polarity by excluding the anterior PARs from the posterior. These findings suggest that PAR-2 plays an essential role in transforming the transient cue from the sperm asters into a stably polarized axis. PAR-2 is a novel protein with a ring finger and an ATP binding site, and we are currently investigating which of these domains are required for function.
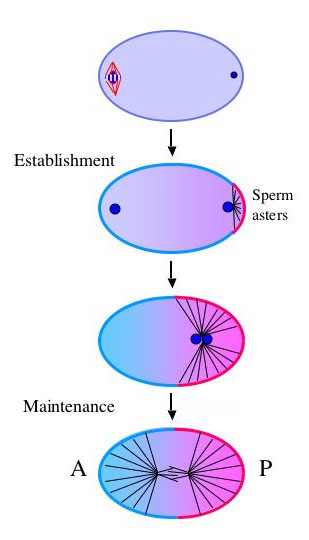
Figure 1
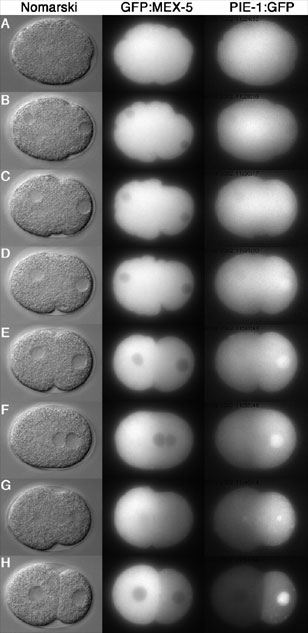
Figure 3
In the case of PIE-1, we identified two complementary mechanisms required for localization: a first (still poorly understood) mechanism which causes PIE-1 to become enriched in the posterior half of the zygote before cleavage, and a second mechanism which degrades any protein left over in the anterior after cleavage. The latter is mediated by a novel E3 ubiquitin ligase, which recognizes a unique CCCH finger motif in PIE-1 (ref 2). Degradation is activated in anterior cells by MEX-5 and MEX-6 and inhibited in posterior cells by PAR-1.
These findings have led us to wonder whether localized protein degradation could also be driving asymmetry in the zygote. I will speculate on the possibility that local changes in protein stability between competing proteins may be the dominant mechanism driving asymmetric localization in C. elegans zygotes.
cytomechanical modules 2003 • back