Gabor Forgacs
Self-assembly is the fundamental process, which generates structural organization across scales both in living and inanimate systems. Morphogenesis, an example of self-assembly characterizes early development when through cell-cell and cell-extra cellular matrix interactions the developing organism and its parts gradually acquire their final shape. Morphogenesis is under strict genetic control. However genes do not give rise to forms and shapes, physical mechanisms do. Therefore it is obvious that physical mechanisms, in particular those typical for viscoelastic materials, must play crucial role in the characteristic self-assembly machinery shaping the embryo.
The present talk is a short overview of biomechanics, the discipline that deals with the viscoelastic properties of biological materials. We will concentrate on early morphogenesis and point out the usefulness of the biomechanical approach to biologists through specific examples. We will present techniques to study biomechanical phenomena from the sub-cellular to the organismal level. Finally, the application of knowledge collected through biomechanics about self-assembly and self-organization will be applied to the evolving technology of organ printing that is in vitro morphogenesis. The talk will be organized as follows:
Embryonic tissues mimic the behavior of liquids. We first overview the experimental evidence suggesting that embryonic tissues in many respect behave as fluids. One manifestation of such behavior is shown in Figure 1, which demonstrates that an initially shapeless aggregate of embryonic cells evolves to a configuration with minimal surface area (i.e. sphere), to minimize its interfacial energy, just as ordinary liquids do in the absence of external forces.
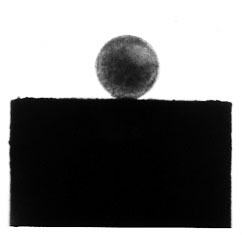
Fig. 1. The final configuration of an aggregate (~400 micron diameter) composed of appr. 40,000 chicken embryonic heart cells.
Consequences of tissue liquidity. Tissue liquidity allows the understanding of a number of early morphogentic phenomena. We will concentrate on sorting, a process in which from an initially random mixture of two (or more) cell populations one eventually nucleates and becomes surrounded by the other as shown in Figure 2. The mechanisms governing both the equilibrium shape and the kinetic approach to this state can be interpreted in terms of fluid mechanics. It will be shown how biomechanical measurements at various scales (using magnetic tweezers, AFM force manipulator, parallel plate compression device) on such systems allow to obtain valuable biologically relevant information.
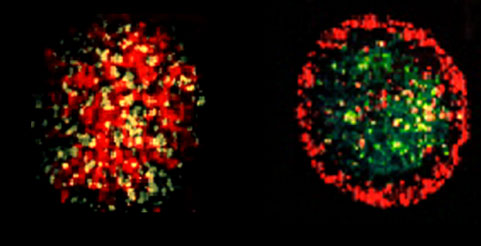
Fig. 2. Left: random initial mixture of two embryonic L cell populations transfected with N-cadherins (the green cells express 33% more cadherin than the red cells, yellow is the consequence of color mixing). Right: the final equilibrium configuration 20 hours after mixing. The linear size of the aggregates is around 200 mm.
Viscoelastic properties of extra-cellular matrices. Without the ECM the morphogenetic program cannot be fully implemented. We will overview the benefits of considering the ECM as a viscoelastic material when trying to tame morphogenetic forces.
Application to organ printing. Organ printing is an evolving technology within tissue engineering. It aims to eventually producing replacement organs in a consistent, made-to-measure manner, without the harmful consequences characterizing most of the presently used similar technologies. We will discuss this technology in some detail, but will mostly focus on how the self-organizing properties of cells and tissues, the basis of morphogenesis and their biomechanical properties, as discussed earlier, aid in the realization of this technology. In particular, we will introduce the notion of bioink and biopaper, the former being the tissue specific spherical aggregates, an example of which is shown in Figure 1 and the latter being the ECM providing the physical environment (i.e. scaffold) for printing. We will also show how computer modeling can help in optimizing this technology. An example of the evolution of a tubular organ structure, from initially printing aggregates along a circle is shown in Figure 3.
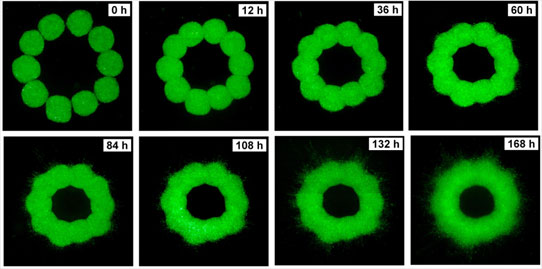
Fig. 3. Time evolution of a ring of 10 printed spherical aggregates (475 micron diameter) in a collagen gel (1.0 mg/ml concentration). Aggregates were delivered with a special bioprinter.
In summary, this talk is aimed at demonstrating the power of biomechanics in understanding and applying principles of morphogenesis.
cytomechanical modules 2003 • back