One of the fundamental engines of metazoan morphogenesis involves the active intercalation of cells along one or more embryonic axes driving tissue convergence along those axes and (necessarily) extension along orthogonal axes. Versions of this convergent extension engine operate across the chordate phylum in a variety of different embryonic contexts and at different times during gastrulation and neurulation to shape the body axis in very different ways. And yet detailed studies of local cell behaviors underlying convergent extension in different organisms suggests a fundamentally conserved mechanism- one in which globally polarized crawling of individual cells across the surfaces of their adjacent neighbors drives progressive cell intercalation.
In the past several decades, we have learned a great deal about the cytoskeletal and adhesive machinery underlying the generation and transmission of motile forces within cells. What has become increasingly clear is that much of this machinery is highly conserved, and furthermore that it is organized into functional modules specialized e.g. for the generation of local protrusive forces; or contractile forces; or for the transmission of such forces between cells, or from cells to external substrata, via local adhesive linkages (see Figure 1).
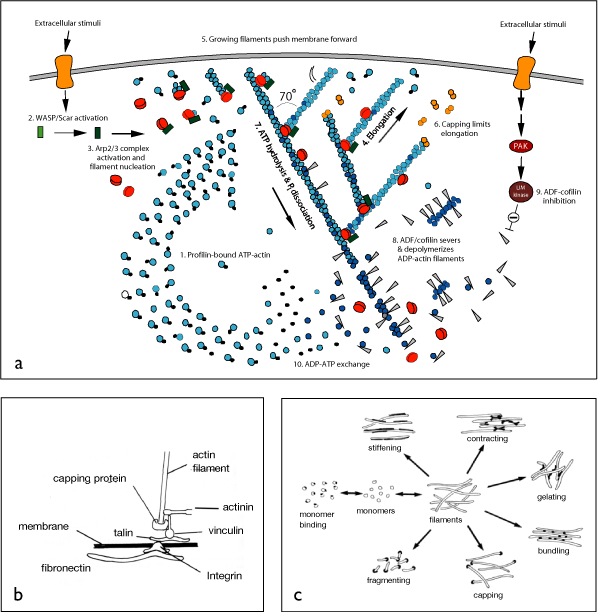
Figure 1. Cytomechanical modules underlying the crawling of animal cells across an external substratum. a) An Actin Polymerization Ram drives localized extension at the leading edge (from Pollard et al 2000). b) Integrin-based Adhesive Linkages mediate the establishment of reversible contacts between cell cortex and substratum and the transmission of force through these contacts (from Bray 2000). c) A Contractile Mesh generates active contractile forces within the cortex that set up a tug of war between points of adhesive contact with the substratum and thereby moves the cell body forward (from Alberts et al 2003).
Such functional modularity has important implications for how we think about specific mechanisms of convergent extension and also about the evolution of animal forms. For example, it suggests that many differences in the morphologies of related embryonic structures may arise through different tunings of the same modular machinery, through spatial or temporal differences in its deployment, or through differences in the mechanical context in which it operates.
However, it has been difficult to address these questions, or to pin down the mechanisms at work in any specific instance of convergent extension, because it has been difficult to make the conceptual leap between local force-generating processes operating at the cellular or subcellular level, and their global tissue level consequences. Physical law dictates that at any moment, local forces arising everywhere within an embryo must resolve themselves simultaneously and globally into instantaneous patterns of movement and deformation. In most cases it is beyond the human brain (or at least my brain) to intuit how they will do so.
One of our major efforts to address these issues has been to develop a computational model that can predict patterns of cell shape change and rearrangement that emerge as a consequence of local forces generated by subcellular morphogenetic modules operating within each of many cells in a close packed tissue. This model was motivated by our experimental work on epithelial cell rearrangements underlying ascidian notochord formation (Munro and Odell 2002a&b) and it builds upon the fundamental assumption, justified by our experimental observations, that embryonic cells intercalate within a close-packed monolayer by crawling across the surface of neighboring cells using the same (or functionally analogous) machinery isolated tissue culture cells use to crawl across an external substratum (Figure 2). We begin with the standard textbook model for how a single cell crawls on an isolated substratum through a combination of three processes, each corresponding to one of the cytomechanical modules shown in Figure 1: 1) localized protrusive extension at the leading edge; 2) the formation of adhesive contacts with the substratum and their linkage to the cortical cytoskeleton; and 3) The generation of contractile forces within the cortex and possibly the deeper cytoplasm that sets up a tug of war between different points of adhesive contact and ultimately moves the cell forward. We build a single model cell from a collection of discrete interconnected mechanical elements, each of which represents a piece of the cortex or internal cytoplasm or an adhesive linkage; and we endow these elements with mechanical and kinetic properties designed to mimic, as closely as possible, those of their real counterparts. We do so in a way that allows us to map experimental measurements of cytoskeletal or adhesive mechanics onto the parameters that govern each element's behaviors.
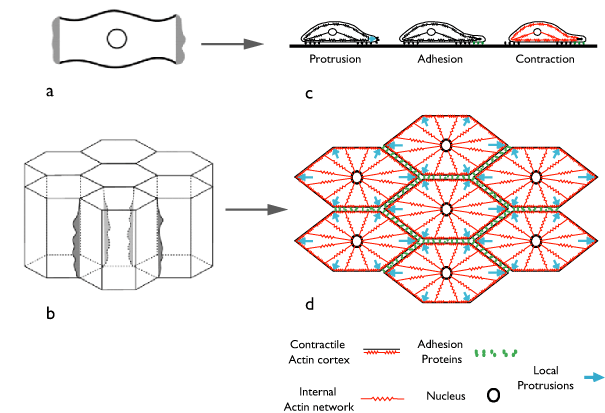
Figure 2. How the same conserved cytomechanics that propel an isolated cell across a rigid external substratum might drive convergent extension of cells within an epithelium. a & b) The basic analogy: An epithelial cell participating in convergent extension (b) crawls across the surface of its neighboring cells in the same way that an isolated cell (a) crawls across an external substratum. In particular, each internal basolateral edge of the epithelial cell is functionally analogous to a leading edge of the isolated cell. c & d) a 2D view showing how we imagine conserved (or in the case of adhesion functionally analogous) cytomechanical modules to be deployed in the isolated motile cell (c) and the epithelial cell (d) during convergent extension.
We have been using this model in a number of different ways. One is to ask whether the same machinery that endows a single cell with the ability to crawl across an isolated substratum could also account qualitatively and quantitatively for the ability of a sheet of close-packed cells to converge and extend (it can). Another is to ask whether the ability to converge and extend is robust with respect to variations in the parameters that govern the behaviors of model elements (it is, suggesting that the real convergent extension engine may be similarly robust to genetic variation in the underlying morphogenetic machinery). More generally, we can ask how patterns of cell shape change and rearrangement depend either on the local cytoskeleletal and adhesive mechanics operating within each cell, or upon global physical constraints arising through contacts with adjacent tissues.
cytomechanical modules 2003 • back