Denis Wirtz
How the cytoskeleton, a heterogeneous network of dynamic filamentous proteins, provides the cell with structural support is not well understood. Particle-tracking methods, which probe local mechanical properties, are well suited to test existing hypothesis derived from in vitro models of reconstituted cytoskeleton networks. My talk will present recent applications of single- and multiple-particle tracking microrheology to cell biological problems, with an emphasis on networks of the semiflexible polymer F-actin. Extensive knowledge of the properties of these polymers allows a rigorous comparison between theory and experiments to a level rarely matched by synthetic polymers.
Cell biology is entering a phase where biophysical methods hold center stage. In this postgenomic era, where genes and gene products of normal and pathological tissues are rapidly separated, identified and classified using high-throughput methods, there is a renewed emphasis on quantitative studies of cell behavior. This brief review focuses on some of the latest developments in colloidal science and polymer physics, which have led to new insight into cell mechanics and into the viscoelastic properties of the cytoskeletal filamentous proteins actin in vitro and in live cells.
Filamentous actin (F-actin) is the polymer form of actin (G-actin; M = 43 kDa), which is expressed in all types of animal and human cells (Fig. 1). F-actin has attracted much attention from physicists, engineers, and biologists alike because of its fundamental role in key cellular functions, including cell migration, cytokinesis, endocytosis, cell adhesion, and because F-actin constitutes an extremely useful model for semiflexible polymers.
Semiflexible polymers constitute a vast and important class of polymers to which belong many molecules of technological and biological importance, including DNA and kevlar. F-actin is considered a semiflexible polymer because it has a mean contour length of L = 15 mm, which is similar to its mean persistence length, Lp = 17 mm. F-actin, a dynamic polymer of G-actin molecules, is uniquely versatile as its fundamental properties, including its mean length, length distribution, and bending rigidity, can readily be manipulated using actin-binding molecules. A key advantage of F-actin over synthetic polymers is that it can easily be visualized in the fluorescence microscope via specific labeling. Versatility and ease of visualization make F-actin an attractive polymer to test not only the predictions, but also the underlying molecular assumptions of dynamic theories of semiflexible polymers.
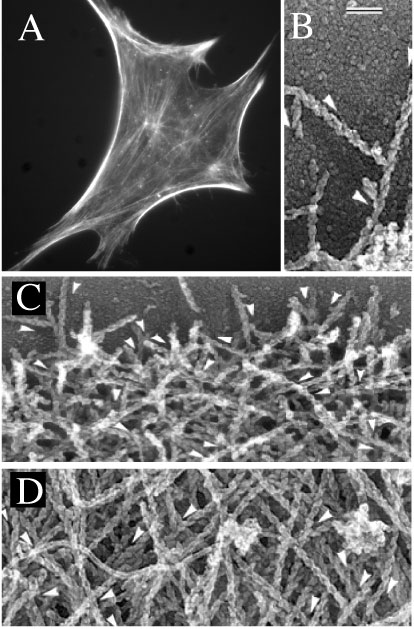
Fig. 1. F-actin organization in cells. a) F-actin organization in a Swiss 3T3 mouse fibroblast obtained by fixing and fluorescence-staining for F-actin. The low side of the figure is 120 mm. b) T junctions between actin filaments at the leading edge obtained by electron microscopy of detergent-extracted fish keratocyte. Bar is 0.1 mm and applies to figures b-d). c) F-actin organizartion is relatively homogenous structures at the leading edge of the cell, the cell side most proximal to the direction of migration. d) F-actin organization becomes more heterogeneous away from the leading edge. Directions of the slow growing ends of some filaments are shown by arrowheads located next to a filament. Figures b-d) are reproduced from the Journal of Cell Biology, 1997, vol 139, pp 397-515 by copyright permission of the Rockefeller University Press.
The rather extensive knowledge of the physical properties of F-actin (L, Lp, etc.) has allowed rigorous comparison between theory and experiments to a level rarely matched by synthetic polymers. Here, we briefly review the dynamical and rheological properties of Factin in dilute, semidilute, and concentrated solutions. In the dilute regime, Brownian dynamics simulations and theory predict that the stress relaxation modulus of semiflexible polymers scales as G(τ)~τ-3/4 at short time scales. In an intermediate time-scale regime, G(τ)~τ-5/4, a regime that is challenging to verify using conventional rheometers due to the small stresses generated in sheared solutions and the fragility of F-actin. Following the method of Dichtl and Sackmann, this intermediate behavior could be tested at a single-molecule level by tethering a small fluorescent particle to the polymer and tracking its Brownian motion with high temporal resolution using quadrant detection.
The onset of elasticity occurs at concentrations as low as ~0.1 mg/ml. Above this concentration, semidilute F-actin solutions have been shown (and used) to verify predictions of recent polymer-physics theories. These experimentally-verified predictions include the existence and concentration-dependence of a plateau modulus at low strain frequencies (Gp~c7/5), the high-frequency-dependence of the elastic and loss moduli (G′(ω)~G″(ω)~ω3/4), the length-dependence of the relaxation time (τR~L3/2), and the concentration-dependence of the tube diameter inside which filaments are confined at short and intermediate time scales (D~c-3/5). Analogous to the Doi-Edwards-de Gennes tube model, these results show that at time scales shorter than the time required for the lateral fluctuations of a test polymer to hit the tube walls formed by polymer entanglements, the dynamics of the test polymer is dominated by its high-frequency bending fluctuations, for which the relaxation modulus is G(τ)~τ-3/4. At intermediate time scales, the test polymer becomes effectively confined by the tube and the relaxation modulus adopts a plateau value, G(τ)~Gp. Beyond the relaxation time, escape from the tube and terminal relaxation of the stress finally occur. Rheological methods show that, in the semidilute regime, F-actin solutions under stress display a slight strain-hardening, where the apparent modulus increases for increasing deformation amplitudes up to 3 - 7%, a behavior also observed in living cells. Past that threshold (i.e. yield strain), F-actin solutions soften rapidly. Theory predicts severe strain-softening at large strain amplitudes, but no strain-hardening at low strain amplitudes. This discrepancy between theory and experiment suggests the existence of (weak) interactions between F-actin polymers. A slightly higher-than-predicted value for the plateau modulus further supports the existence of non-steric interactions between filaments, may be due to electrostatic interactions.
Above a concentration of ~2 mg/ml, actin filaments form a liquid crystalline phase due to the local rigidity of F-actin. Concentrated solutions of F-actin display a low plateau modulus, Gp =30-50 dyn/cm2, which is independent of concentration beyond ~2-3 mg/ml. In contrast, microrheological measurements reveal a mean modulus ~820 dyn/cm2 in the actin-rich cortex of the cell, where actin concentration is ~100 mg/ml actin. Hence, solutions containing physiological concentrations of actin have a stiffness much lower than observed in living cells. Moreover, a cell exposed to highly specific actin-depolymerizing drugs softens dramatically, an effect accompanied by the rapid contraction of actin-rich cell extensions. F-actin's contribution to cell mechanical integrity is therefore essential but not sufficient.
Our knowledge about F-actin behavior in dilute, semidilute, and concentrated solutions sets the stage for the study of more complex, and indeed more physiological, structures where F-actin is crosslinked into stiffer architectures by crosslinking/bundling proteins. More than 60 actin-binding proteins have been identified in mammalian cells, among which seven are F-actin crosslinking/bundling proteins, proteins that mediate the formation of both orthogonal networks and ordered bundles. F-actin crosslinking/bundling proteins appear, sometimes concurrently, in specialized subcellular complexes, including lamellipodia, filopodia, stress fibers, and focal adhesions, which coordinate cell migration and cell spreading. The flexible homodimeric actin-binding protein α-actinin stands out as an archetype of such actin crosslinking/bundling protein. F-actin/α-actinin solutions exhibit elastic moduli that nearly match those found in live cells. Because of its intrinsic flexibility, α-actinin crosslinks F-actin into orthogonal networks at low concentrations and into loose filament bundles at high concentrations. An increase in the temperature-modulated binding lifetime enhances both the plateau modulus and the hardening effect of F-actin networks under shear strains. However, the microheterogeneity of F-actin networks greatly increases for increasing α-actinin concentration. These two effects, variable binding lifetime and network microheterogeneity, are not explicitly incorporated in current models of F-actin networks.
The recent introduction of particle tracking microrheology (PTM) has opened a realm of possibilities in the characterization of novel materials and cell biological systems. Because it requires a small sample, PTM can readily be used for high-throughput screening of novel/expensive materials, such as specialty polymers, and biological specimen, such as lung mucus, synovial fluid, and blood. Unlike most rheometers, PTM can reliably probe low viscosity samples with exquisite sensitivity. Moreover, PTM provides quantitative information about the degree of heterogeneity of complex fluids.
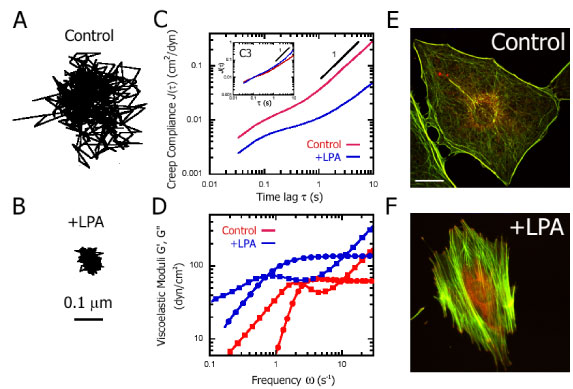
Figure 2. Cytomechancal response of Swiss 3T3 cells to Rho activation. A and B, Typical trajectories of nanospheres injected into the cytoplasm of serum-starved Swiss3T3 cells before (A) and 15 minutes after (B) treatment with 1 µg/ml LPA. Scale bar indicates length scale for both trajectories. C, Ensemble-averaged creep compliance directly computed from the mean-squared displacements of the thermal motions of nanospheres injected into the cytoplasm of serum-starved Swiss 3T3 cells before (red) and 15 minutes after (blue) treatment with 1 µg/ml LPA. (Inset), Ensemble-averaged creep compliance of the cytoplasm of serum-starved Swiss 3T3 cells injected with 10 µg/ml C3, before (red) and after (blue) treatment with 1 µg/ml LPA. D, Frequency-dependent viscous and elastic moduli, G″(£s) and G′(£s), obtained from ensemble-averaged creep compliances (C). G′(£s) is represented by circles and G″(£s) by squares. E and F, Fluorescent micrographs of serum-starved Swiss 3T3 cells before (E) and 15 minutes after (F) treatment with 1 µg/ml LPA. Actin filaments (green) were visualized with Alexa 488 phalloidin, while focal adhesion complexes (red) were visualized with an antibody for vinculin and Alexa 566 goat anti-mouse. Scale bar represents 20 µm.
PTM tracks the thermally-excited fluctuations of individual probe particles imbedded in a complex fluid to probe its local viscoelastic properties. The time-lag dependence mean squared displacement (MSD) of each particle, <Δr2(τ)> = <[x(t+τ)-x(t)]2+[y(t+τ)-y(t)]2>, which is computed from the time-dependent coordinates of that particle, [x(t), y(t)], reveals the viscoelastic nature of the microenvironment in the immediate vicinity of each particle. If the MSD scales with time lag (<Δr2(τ)> ~ t), then the microenvironment is liquidlike over seconds for laser-deflection tracking and < 1 min for video-tracking. In this case, the random viscous stresses created by the fluctuations of the particle are rapidly dissipated. If the MSD grows more slowly than time (<Δr2(τ)> ~ tα where α <1), the microenvironment is partially elastic. The random forces created by the fluctuating particle are met with restoring forces of equal or smaller magnitude. Faster-than-linear growth of the MSD signals either convection of the specimen or, more interestingly, directed transport via motor proteins, which actively move actin filaments in a network or bind organelles intracellularly. The MSD can either be transformed into a creep compliance trace, Γ(τ)=(πa/kBT)<Δr2(τ)> (a is the radius of the probe particle, T is the temperature of the specimen, and kB is Boltzmann constant), or converted into frequency-dependent viscoelastic moduli via Laplace/Fourier transformation.
The motion of particles imbedded in a complex fluid or in a living cell can be monitored either by imaging or non-imaging methods. Laser deflection/quadrant detection is the (non-imaging) method used for the first proof of principle of PTM. The motion of a single particle deflects the incident beam of a low-power laser; the scattered light is collected by a quadrant photodiode detector, mounted onto a light microscope. Laser deflection/quadrant detection allows remarkable spatio-temporal resolution: ~1 nm displacements with 20 kHz time resolution are measured routinely. Therefore, local viscoelastic moduli spanning about 5 decades in frequencies are measured in seconds. This superior resolution has been exploited to probe the in-plane and out-of-plane shear elastic moduli of actin/vesicle complexes, actin dynamics in red blood cell's spectrin-actin network, and the local viscoelastic properties of an adherent cell by tracking the displacements of intracellular lipid-storage granules. These live-cell PTM measurements showed that the actin-rich cytoplasm exhibits elastic and loss moduli that scale as G′(ω)~G″(ω)~ω3/4, suggesting a viscoelastic character dominated by semiflexible polymers. PTM also demonstrated that subcellular regions of cytoplasm, the lamella and the perinuclear region, exhibit large difference in stiffness, a conclusion that could not have been reached using traditional cell-mechanics methods, which are typically limited to global measurements of cell stiffness.
The motion of particles imbedded in a complex fluid or in a living cell can be monitored either by imaging or non-imaging methods. Laser deflection/quadrant detection is the (non-imaging) method used for the first proof of principle of PTM. The motion of a single particle deflects the incident beam of a low-power laser; the scattered light is collected by a quadrant photodiode detector, mounted onto a light microscope. Laser deflection/quadrant detection allows remarkable spatio-temporal resolution: ~1 nm displacements with 20 kHz time resolution are measured routinely. Therefore, local viscoelastic moduli spanning about 5 decades in frequencies are measured in seconds. This superior resolution has been exploited to probe the in-plane and out-of-plane shear elastic moduli of actin/vesicle complexes, actin dynamics in red blood cell's spectrin-actin network, and the local viscoelastic properties of an adherent cell by tracking the displacements of intracellular lipid-storage granules. These live-cell PTM measurements showed that the actin-rich cytoplasm exhibits elastic and loss moduli that scale as G′(ω)~G″(ω)~ω3/4, suggesting a viscoelastic character dominated by semiflexible polymers. PTM also demonstrated that subcellular regions of cytoplasm, the lamella and the perinuclear region, exhibit large difference in stiffness, a conclusion that could not have been reached using traditional cell-mechanics methods, which are typically limited to global measurements of cell stiffness.
Laser deflection/quadrant detection has limitations as it allows tracking of only 1 or 2 particles at a time, which prevents real-time mapping of local viscoelastic properties. Moreover, only displacements smaller than ~0.3 mm can be monitored; hence particles imbedded in soft gels cannot be probed for a long time as their excursions step outside of detection. Multiple particle microrheology resolves many of these issues without much compromise in spatial resolution. The displacements of individual fluorescent or refractive particles are monitored simultaneously using either a CCD or SIT camera, typically at video rate (30 - 60 Hz). This imaging method retains a great spatial resolution of the displacements, ~5 nm, by tracking the centroid of the diffraction-limited image of each probe microsphere in the field of view. A key advantage of multiple particle tracking is that the shape of the distribution of the MSDs (or viscoelastic moduli) characterizes the degree of heterogeneity of the solution. For instance, the MSD distribution in homogenous solutions of polyethylene-oxyde or glycerol is symmetric and narrow; but becomes asymmetric and wide in F-actin networks and in cells. A practical method to analyze MSD distributions is to compare the skewness and kurtosis of the MSD distribution with those of the corresponding Gaussian distribution of same mean and standard deviation. An alternative method is to compute the contributions of the 10%, 25%, and 50% highest MSD values to the ensemble-averaged MSD, which are larger than 10%, 25%, and 50% for an inhomogenous solution. This type of analysis allows for the comparison of MSD distributions that encompass different MSD values as shown for F-actin and keratin.
Time-resolved multiple particle tracking, which monitors spatio-temporal variations of the microrheological properties in complex fluids, was recently used to uncover the underlying mechanism of F-actin gelation. A 1mg/ml actin solution forms an overlapping polymer network in ~ 1 min; yet the same solution takes ~ 1 h to reach a steady state elasticity. This slow gelation of F-actin is hypothesized to be due to the slow establishment of an homogeneous network. To test this model, time-resolved multiple particle tracking microrheology is introduced: the thermally-excited motion of hundreds of particles imbedded in an F-actin solution undergoing gelation is monitored in time and space. Time-resolved multiple particle-tracking shows that the degree of heterogeneity of an F-actin solution undergoing gelation decreased with time, tracking gelation kinetics. An increasing actin concentration increases the rate of actin polymerization, but decreases the rate of gelation and the rate of homogenization of F-actin solutions.
Another application of time-resolved multiple particle tracking microrheology consists in monitoring the spatio-temporal changes in cells subjected to pharmacological treatments or growth factors and LPA. Fig. 2 illustrates this application: serum-starved Swiss 3T3 fibroblasts are exposed to LPA, a small molecule that activates the small GTPase Rho and re-organizes the actin network.
Despite quantitative agreement between mechanical and particle tracking measurements for many colloidal and polymer solution systems, questions remain regarding the analysis of PTM experiments. The thermally excited motion of a probe particle imbedded in a biopolymer network may create a depletion zone, which could artificially decrease the apparent stiffness of the network. Moreover, the surface chemistry of the probe particle can affect the measurement of the mechanical properties of the network. Finally, local micro-heterogeneity of the network may greatly influence the estimation of the overall elasticity. These issues need to be addressed to refine current livecell particle-tracking micro-rheometers. Two-point microrheology suggests that the measurement of the pair-correlation of displacements could yield micro-mechanical parameters that are independent of the surface chemistry and size of the probe particles. One-point and two-point microrheology analysis of particle displacements yield different mechanical properties in F-actin. For a 1mg/ml F-actin solution, two-point correlation analysis yields elastic and viscous moduli that are very similar, G′≈G″≈2 dyn/cm2 at 1 rad/s, which corresponds to a phase shift of 45°. Moreover, G′≈G″~ω1/2, which would imply that, at short time scales, F-actin resembles the stress relaxation dynamics of a flexible polymer. In startling contrast, one-point microrheology analysis shows a distinct plateau modulus at low frequencies (≈2-10 dyne/cm2), a phase shift of δ ≈25°, i.e. an F-actin network behaves like a viscoelastic solid, and G′~G″~ω3/4 at high frequencies, in agreement with magnetic-bead rheometry measurements. This suggests that methods involving forced bead movement and one-point microrheology measure an aspect of F-actin dynamic response which is different from, potentially complementary to, that measured by two-point microrheology.
Cytoskeleton microrheology is in its infancy, but particle-tracking methods are particularly well-suited to fill the gap between in vitro and in vivo systems. Many advances need to take place in theory, experimental methods, and choice of systems. A first step in that direction is to reconstitute more physiologically-relevant architectures, such as the dendritic actin structures mediated by the actin-binding protein Arp2/3 observed at the leading edge of a cell (Fig. 1b). Another is to investigate the synergistic potential of cytoskeletal polymers and crosslinking proteins (Fig. 1). Thoretical models of cytoskeleton micromechanics need to incorporate the finite lifetime of binding and the intrinsic flexibility of crosslinkers, nonsteric interfilament interactions, as well as network microheterogeneity. New optical tools need to be developed, which would combine the exquisite temporal resolution of laserdeflection and the superior statistics offered by video multiple-particle tracking.
cytomechanical modules 2003 • back