Although it is certainly true that microtubules and actin play distinct roles in cells, it has been evident for some time that interactions between these seemingly distinct filament systems exist. Vasiliev hinted at this years ago when he showed in migrating fibroblasts that maintenance of the polarized distribution of actin-dependent protrusion at the leading edge required an intact microtubule cytoskeleton. Microtubules and actin exhibit two mechanistic classes of interactions, "Regulatory Interactions" in which Rho-family signaling cascades co-regulate microtubules and actin, and whose activity is spatiotemporally controlled by microtubules, and "Structural Interactions" in which actin and microtubules are physically cross-linked. RhoA mediates formation of contractile actin bundles and microtubule stabilization, Rac1 activates polymerization of both actin and microtubules to promote lamellipodial protrusion, while Cdc42 mediates actin bundle polymerization into filopodia and directs the positioning of the microtubule organizing center (MTOC) in migrating cells. At the same time, microtubule disassembly activates RhoA while microtubule assembly promotes Rac1 activation. Recent in vivo and in vitro studies show that structural interactions between microtubules and actin result in an interdependence of their dynamic organization in migrating cells. Analysis of actin and microtubules by dual-wavelength FSM showed that microtubules are coupled to actin retrograde flow in the lamella, and to anterograde motion of actin in the cell body (Figs. 1,2). Microtubules also grow along actin bundles towards focal adhesions, and microtubule ends often are dragged through the cytoplasm by their connection to moving actin bundles.
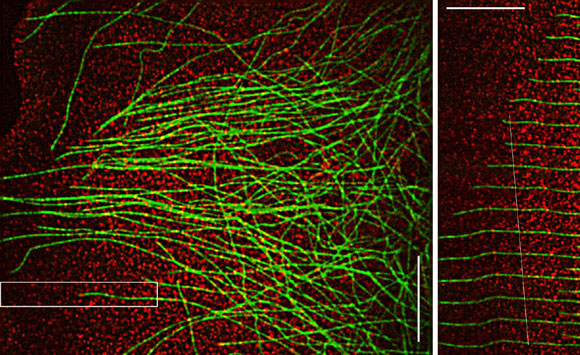
Fig 1. Microtubules and F-actin movements are coupled in the lamellum of a migrating newt lung epithelial cell. (A) FSM image, leading edge is at the left. (B) Time montage of the boxed region in the upper panel. The microtubule is transported rearward while simul-taneously growing towards the leading edge. The white line tracks a speckle on the microtubule, which is moving at the same velocity as speckles in the actin meshwork. Bar = 10 mm.
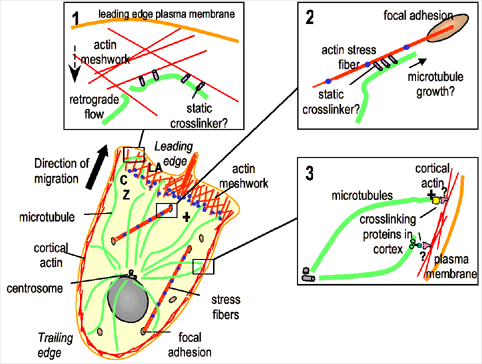
Fig. 2. In migrating tissue cells, microtubule (green) minus ends are organized by the centrosome, which is positioned between the nucleus and the leading edge, and their plus ends are toward the leading edge. In lamellipodia (LA), F-actin (red) is in a meshwork that undergoes retrograde flow towards the convergence zone (CZ) where myosin contraction (blue dots) is concentrated. Stress fibers are contractile acto-myosin bundles with their ends anchored in focal adhesions. Insets: putative microtubule-actin interactions in different regions of a migrating cell. (1) In the lamellum, microtubules are coupled to F-actin undergoing retrograde flow, compressing and breaking microtubules to promote regional microtubule turnover. (2) Microtubules could target focal adhesions by cross-linking to and growing along focal adhesion-associated actin bundles. (3) Microtubule plus ends may be anchored at the cell cortex via interactions between plus-end binding proteins and actin binding proteins to orient the MTOC towards the direction of migration.
How do these interactions mediate cell movement? One hypothesis is that cell motility depends on the structural linkage of microtubules to actin retrograde flow, which in turn establishes and maintains a signaling gradient that perpetuates motility. Constant microtubule retrograde flow requires compensatory net growth towards the leading edge. Behind the lamellum, microtubule breakage and depolymerization occurs as a result of the compressive forces of the contracting actin to which they are bound. Thus, linkage of microtubules to regional actin movements results in a gradient of microtubule assembly states in the cell with growth at the leading edge and shortening behind the lamellum. Microtubule growth could promote local activity of Rac1 in the cell front to drive lamellipodial protrusion and perpetuate further microtubule growth, while microtubule shortening could activate RhoA behind the lamellum to drive actomyosin contraction and promote the stabilization of a subpopulation of microtubules, possibly to protect them from breakage and thus maintain the overall polarization of the microtubule cytoskeleton. We call this the "polymerization/contraction treadmill module" (Fig 3a) and we will discuss how it appears to be conserved for use in other cellular environments where gradients in polymerization and contraction must be perpetuated.
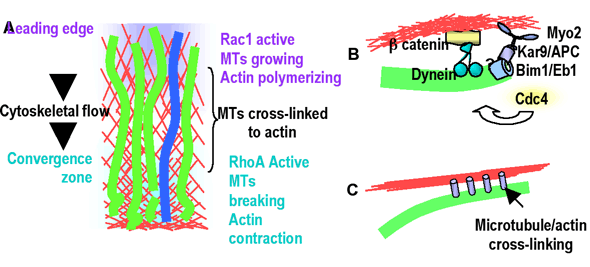
Fig. 3. Microtubule-actin interaction "activity modules." (A) The "polymerization/contraction treadmill" module. At the leading edge Rac1 activity stimulates actin and microtubule polymerization, and is perpetuated by microtubule growth. Microtubules (green) crosslinked to actin (red) undergo retrograde flow. As they approach the region of actin contraction, they buckle, break and depolymerize. Microtubule disassembly activates RhoA to perpetuate contraction and stabilization of a subset of microtubules (blue). (B) The "plus end/cortex anchor" where stable attachment of microtubules to the actin cortex via a plus-end complex is directed by Cdc42. (C) The "actin bundle/microtubule guidance" module may be important for precise positioning of microtubules at focal adhesions.
Alternatively, structural and regulatory microtubule/actin interactions may mediate specific spatiotemporal regulation of focal contacts with the substrate to guide cell motility Microtubules may bind to and grow along actin filament bundles to precisely deliver a signal that promotes focal adhesion disassembly (Fig 2.2). We call this the "actin bundle/microtubule guidance module" (Fig 3c), and will discuss how it may be used in situations where the targeting of single microtubules to precise positions is required.
Finally, microtubule/actin interactions could orient microtubules towards the leading edge, which could direct the delivery of signaling molecules or membrane components required for lamellipodial protrusion. Recent evidence suggests that Cdc42-mediated MTOC reorientation may occur by "capture" of microtubule ends by the actin cortex, where cytoplasmic dynein motors pull the cytoplasmic microtubule complex into position. We call this the "plus end/cortex anchor module" and will discuss how it may be conserved in processes that require asymmetric microtubule distributions in a variety of cellular contexts and functions. (Fig. 3b).
cytomechanical modules 2003 • back