W.M. Bement, C.A. Mandato, and H.A. Benink
Cells and tissues display an astonishing array of outwardly different motility phenomena, including cell division, morphogenesis, and directed cell locomotion. Surprisingly, however, the same subset of players are involved in most, if not all of these processes: microtubules, actin filaments (F-actin), and motor molecules. To understand how identical molecules can control such apparently divergent events, we have sought to characterize basic interactions among microtubules, F-actin, and motors in several "stripped down" systems and processes. These systems, derived from Xenopus oocytes and eggs, include cell free extracts (1-3), inducible cortical flow in oocytes (4,5), and wound healing in oocytes (6-8). Each of these systems has specific strengths that simplify the analysis of interactions among the different cytoskeletal players, and each has been extremely useful in revealing conserved mechanisms that link these systems.
In this presentation, we will focus on our recent findings from the wound healing system. Following damage to the plasma membrane, Xenopus oocytes rapidly assemble a ring-like array of actin filaments and myosin-2 around the wound that is surrounded by radially-organized microtubules (Fig. 1; refs. 6-8). This array closes over time, and in so doing, drives extrusion of the damaged cell surface into the surrounding medium.
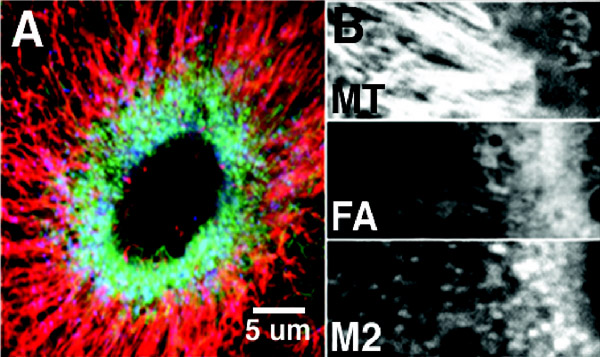
Fig. 1. A. Triple label showing microtubules (red), F-actin (green) and myosin-2 (blue) around wound made in a Xenopus oocyte. The microtubules are organized into a radial array that encloses the ring of F-actin and myosin-2B. Close up of the wound edge, showing microtubules (MT), F-actin (FA) and myosin-2 (M2) separately. Most of the microtubules terminate near the edge of the region of highest F-actin and myosin-2 density. Taken from reference 8.
4D imaging using a combination of fluorescent probes and specific manipulations of the cytoskeleton has shown that this array is generated both by assembly of actin filaments and myosin-2 in specialized zones at wound borders, as well as by myosin-driven contraction of stable F-actin to the wound border (ref. 7). Microtubules are transported to the wound borders as a result of association with moving F-actin (Fig. 2) and are also assembled at the wound border independently of the actin cytoskeleton (ref. 8).
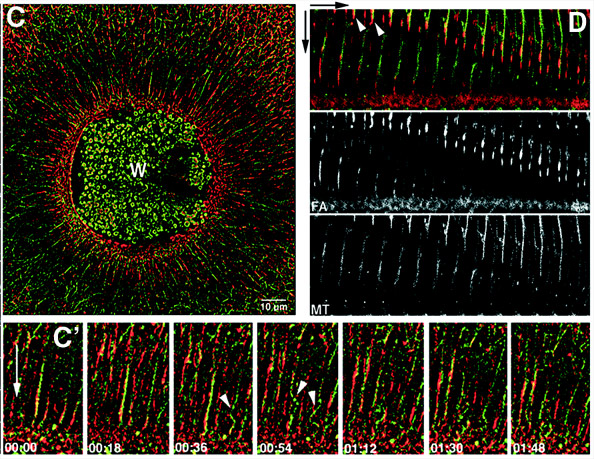
Fig. 2. Images from 4D movies showing transport of microtubules (green) by F-actin (red) during wound healing. C is a low mag picture provided for orientation while C' and D provide higher magnification views of the transport process. Microtubules move toward wound (downward arrows) in association with F-actin (C', D) and buckle as they hit the actin-rich region at the wound edge (arrowheads, C'). Taken from reference 8.
Just as the recruitment of microtubules to the wound edge is in part due to actomyosin-based motility, the microtubules, in turn, control the local assembly of actin and myosin-2, generating a positive feedback loop. This control is exerted at least in part by microtubule-dependent control of the rho GTPases rhoA and Cdc42, as shown by the fact that RhoA and Cdc42 are rapidly activated in concentric zones around wounds and perturbation of the microtubule cytoskeleton alters the activation pattern of these GTPases (H.A. Benink and W.M. Bement, unpublished results).
Many of the processes observed during oocyte wound healing are consistent with proposed models of cytokinesis (9), and bear remarkable similarity to those occurring during cell locomotion (10), providing strong support for the notion that diverse cell motility events can be underlain by conserved cytoplasmic activity modules (Fig. 3). In this case, the activity module is comprised of actomyosin-dependent transport of microtubules toward the wound edge, complemented by microtubule-dependent assembly of F-actin and myosin-2 around wound borders.

Fig. 3. Schematic showing wound healing "activity module". Taken from ref. 10
cytomechanical modules 2003 • back