(David Velásquez-Carvajal)
Neural tube closure in ascidians (Urochordata) occurs by unidirectional zippering together of epithelial and neural tube cells. Abstracts from other class members provide evidence for several cellular processes that could drive neural tube closure (see in particular S. Semon, M. Montgomery and D. White abstracts). These results suggested a delicate association between contractility of the acto-myosin meshwork and protrusive activity.
First, we demonstrated that actin becomes organized into a purse-string-like, continuous cable that surrounds the neural tube (figure Semon1). Second, we found that active (phosphorylated) myosin specifically accumulates a few cells ahead of the zipper’s leading edge – a structure we refer to as the “V” - (figure Semon4), thereby locally fine tuning cortical contractility. Finally, protrusive activity along each side of the closing neural and epidermal cells filopodia is activated around the zipper (movie 1, also S. Semon's abstract). We propose that filopodia generated locally (local protrusion) reach and adheres to the other side of the “V” (local adhesion), pulling the edges together and eventually making new adhesions. In parallel, we also suggest that these filopodia trigger local activation of contractility, possibly by inducing by myosin phosphorylation in the region “V” (local contractility).
Using chemical inhibition of myosin phosphorylation, we proved that increased local contractility is required for proper neural tube closure (see S. Semon's abstract. However not all mechanisms involved in neural tube closure are amenable to experimental perturbation. Therefore their precise role and respective importance remains unclear. We used a two-dimensional computational model in which individual cells could exhibit these behaviors to varying degrees (figure 1 – based on slide 3) in order to explore their relative contributions (software adapted from Morphogenie).
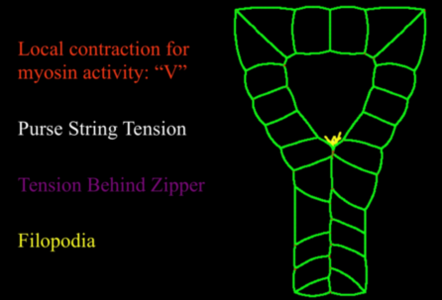
Figure 1: Rules governing the model. We included 4 types of rules: local increased contractility ahead of the zipper (red).
We found that a combination of localized “V” contractility and filopodial action best reproduced the shape changes observed in real embryos (movie 2). In contrast, uniform purse-string contractility failed to form a long tube (movie 3 - a, b, c), while filopodial action alone was ineffective at sealing the tube (movie 4 - a, b, c).
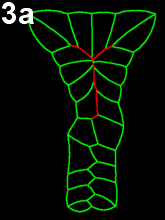
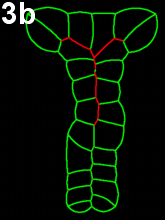
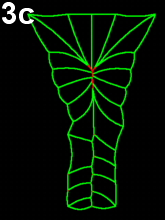
Movie 3 a-b-c: Purse string alone fails to reproduce zippering. Compare with the previous results (Movie 2), modeling neural tube closure solely based on a purse string mechanism failed to properly reproduce the process. The model runs fall in two categories. In movie 3a, corresponding to first type of outputs, cells behind the zipper design an abnormal z-shape, while in Movie 3b-c, corresponding to the other type, neural tube closes by cinching (symmetric closure) and not by posterior to anterior zippering.
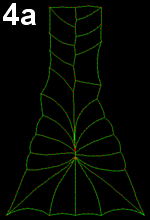
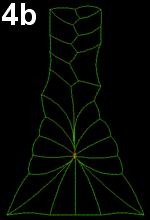
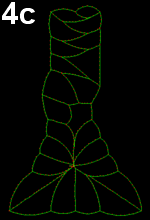
Movie 4 a-b-c: Filopodia alone fail to reproduce zippering. Implementing of filopodia alone could not reproduce neural tube closure by zippering: successful runs were systematically abnormal in some ways. In Movie 4-a, note the pulling of posterior cells, in Movie 4-b pulling of posterior cells and symmetrical cinching, and in Movie 4-c, z-shaped boundary between the two sides of epidermal cells behind the zipper (similar to Movie 3-a).
Localized “V” contractility could drive normal closure kinematics (movie 5), but only for a very restricted set of parameter choices. Further, the model was able to reproduce an experimental observation from partially ablated embryos, in which the “V” had a wider angle than under normal conditions and neural tube closure failed (movie 6, movie 7).
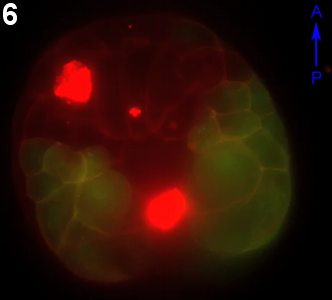
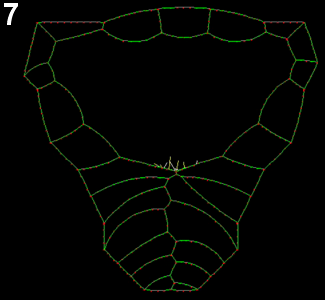
Movie 6: Effect of ablation of anterior-most region of the neural plate. Zippering is properly initiated, but does not progresses the anterior half of the embryo, though apparently cell crawling is not abolished.
Movie 7: Modeling of different geometry as induced by ablation of the anterior neural reproduces the same effect: in conditions that would lead to closure in normal geometry, the process seams to be withheld by the large angle between closing epithelial sheets.
Combinations of various cell behaviors and tissue geometries are needed for any given morphogenetic process to occur properly, and we show here that computational modeling provides a means, complementary to experiments, to identify key features essential to such processes and to explore their respective roles.